- #1
maistral
- 240
- 17
- TL;DR Summary
- What is a propensity function? And some other questions.
I'm trying to simulate a simple series reaction stochastically using Gillespie's algorithm. I found this file:
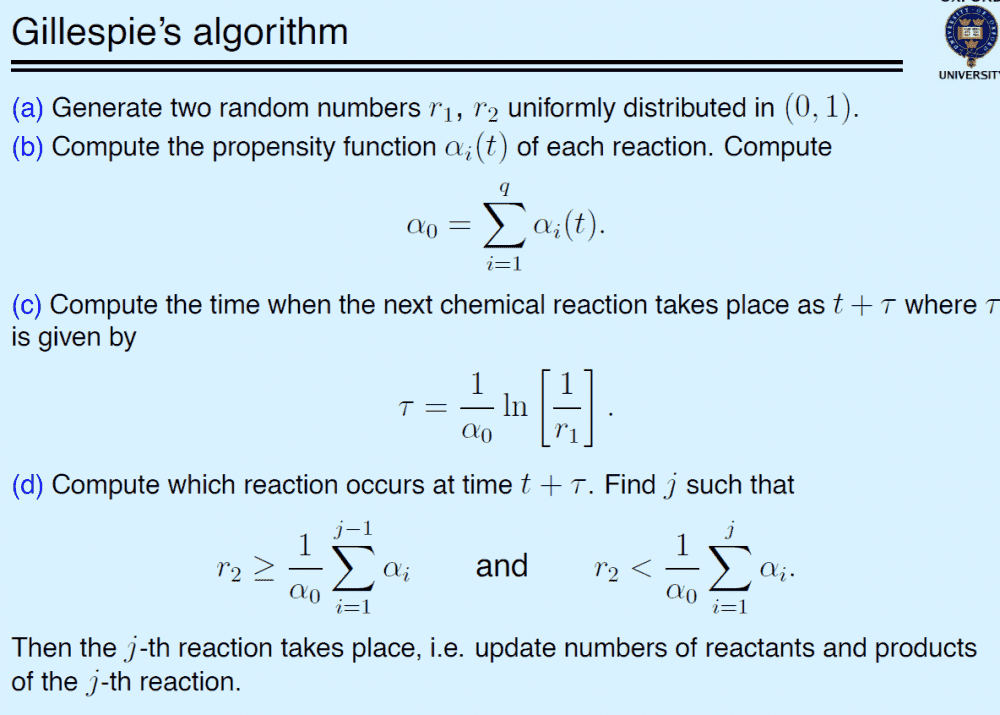
Thank you very much.
- What is this 'propensity function'? Say for example I have the simple reactions:
A --(k1)--> R
R--(k2)--> S
are these 'propensity functions' the rates (a wild guess)? I mean;
α1 = k1[A]
α2 = k2[R],
where [A] and [R] are the number of molecules of A and R? - I am assuming that these 'rate constants' are based on the number of molecules themselves, and not the 'molarity'. I also read from an article (Stochastic chemical kinetics by Lecca) that they're supposed to be different (the rate constants based on the molarity are 'continuous' or something). How do I convert the rate constants from the molarity-based ones to the number of molecules-based ones?
- With regards to the updating of the numbers of reactants and products, assuming the simple reactions
A --(k1)--> R
R--(k2)--> S
are taken into consideration, and that the first reaction is supposed to be occurring, am I correct to assume that I subtract one molecule A and add one molecule R?
Thank you very much.