- #1
ugenetic
- 50
- 3
From some literature, I read the following band structure of the Al doped Si.
The explanation offered: "have vacant acceptor levels at energy Ea above the valence band. Electrons from the top of the valence band can be easily excited into these levels."
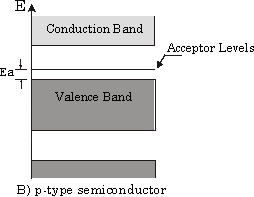
I thought the above explanation is either wrong or counter intuitive.
My own thought:
although Al atoms are injected into the Si lattice, my uneducated guess is that, this block of Si-Al solid probably retained much of the tetrahedral structure, so valence band structure did not change that much compared to a pure block of Si. So the new valence band of Si-Al will have similar width and energy height compared to a valance band of a pure block of Si (for example, if orginal Si's valence band was 10ev high, and 1ev wide, then Si-Al's valance band is probably 9ev high and 0.9ev wide, not much difference)
But now, In Si-Al you will have less total electrons in the solid, so valence Band will have some empty spaces instead of being fully filled, Allowing minority carrier - electron, from other metals or n-types to drop in and recombine. If the recombination is too slow, and you have enough electrons and enough voltage persvasion, even minority carriers can conduct thru the p-type, thus the explanation for npn's pn junction.
If I was right, the the short version of my thought would be: Valence bands of the electrons in Si and Si-Al are similar, but fermi levels of electrons in Si and Si-Al are different, Si-Al's fermi level is lower due to less electrons. allowing empty valence states to exist
The explanation offered: "have vacant acceptor levels at energy Ea above the valence band. Electrons from the top of the valence band can be easily excited into these levels."
I thought the above explanation is either wrong or counter intuitive.
My own thought:
although Al atoms are injected into the Si lattice, my uneducated guess is that, this block of Si-Al solid probably retained much of the tetrahedral structure, so valence band structure did not change that much compared to a pure block of Si. So the new valence band of Si-Al will have similar width and energy height compared to a valance band of a pure block of Si (for example, if orginal Si's valence band was 10ev high, and 1ev wide, then Si-Al's valance band is probably 9ev high and 0.9ev wide, not much difference)
But now, In Si-Al you will have less total electrons in the solid, so valence Band will have some empty spaces instead of being fully filled, Allowing minority carrier - electron, from other metals or n-types to drop in and recombine. If the recombination is too slow, and you have enough electrons and enough voltage persvasion, even minority carriers can conduct thru the p-type, thus the explanation for npn's pn junction.
If I was right, the the short version of my thought would be: Valence bands of the electrons in Si and Si-Al are similar, but fermi levels of electrons in Si and Si-Al are different, Si-Al's fermi level is lower due to less electrons. allowing empty valence states to exist
