- #1
JohnJ
- 4
- 0
- Homework Statement
- A 1.05 L bottle of a carbonated soft drink contains 1.0 L of drink as is pressurized at 5 atm with
CO2 gas. At this pressure, the CO2 concentration in the drink is given by Henry’s law,
C = 0.031 P_g , where C is the concentration in moles/L and P_g is the partial pressure of the gas
(5 atm).
(a) What is the chemical potential of the CO2 in this system relative to the gas at atmospheric
pressure?
(b) The cap is briefly loosened, so that the gas comes fully into equilibrium with the atmosphere,
but the dissolved CO2 remains in solution. The bottle is then sealed.
What are the chemical potentials of the CO2 in the drink and the gas above?
(c) Finally, the bottle is shaken, such that the contents come into equilibrium. Describe what
happens, and calculate what is the pressure inside the bottle now?
Approximately how many times can this process be repeated before the drink goes flat?
- Relevant Equations
- Raoult's Law, Dalton's Law, Equations relating to chemical potentials
For part (a), I used this formula
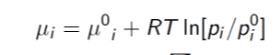
where where the i's represent the substance being used and mu_i^0 represents some reference potential. However, to my knowledge this simply calculates the change in chemical potential from one state to another which is not of much help in finding the relative chemical potential of the gas and the liquid. So I think my answer to (a) is wrong.
For (b) I'm completely lost. I'm not sure if now we take it that the gas is in equilibrium with the atmosphere but is now not in equilibrium with the drink. So then the liquid in the drink and gas in the drink are different temperatures. I'm not sure what new information I can extract from the fact that the gas has come into thermal equilibrium with the atmosphere.
Thanks,
where where the i's represent the substance being used and mu_i^0 represents some reference potential. However, to my knowledge this simply calculates the change in chemical potential from one state to another which is not of much help in finding the relative chemical potential of the gas and the liquid. So I think my answer to (a) is wrong.
For (b) I'm completely lost. I'm not sure if now we take it that the gas is in equilibrium with the atmosphere but is now not in equilibrium with the drink. So then the liquid in the drink and gas in the drink are different temperatures. I'm not sure what new information I can extract from the fact that the gas has come into thermal equilibrium with the atmosphere.
Thanks,