- #1
Lotto
- 214
- 12
- TL;DR Summary
- When we have a Raman spectrum of a molecule, on the horizontal axis, there are values of wavenumbers. What does this wavenumbers correspond to?
I don't know whether it is an energy of a photon emitted by a deexciting molecule, or if it is an energy of laser's photons. Here is an example of such spectrum:
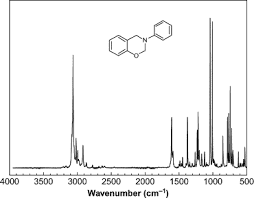
For example, that value of wavenumber ##3000\, \mathrm {cm^{-1}}## is an energy of an emitted photon or a photon from laser? And that high instensity is there why? It means that when the molecule absorbs the photon, it then emits light of a higher intesnity?
For example, that value of wavenumber ##3000\, \mathrm {cm^{-1}}## is an energy of an emitted photon or a photon from laser? And that high instensity is there why? It means that when the molecule absorbs the photon, it then emits light of a higher intesnity?