- #1
Boon Jie
- 3
- 1
Hi all,
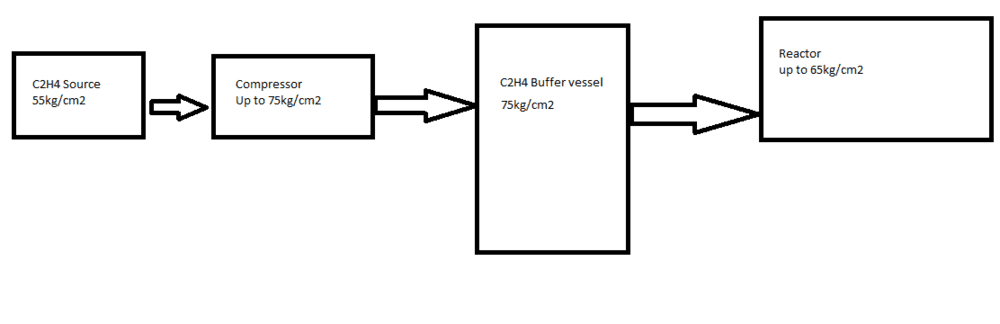
I would like to ask why is there ice formation on the exterior of the pipes between compressor to reactor when there is no flow passing through
or what kind of phenomenon is this?
My colleagues have suggested PV=nRT and adiabatic process as the possible reasons but I still feel that something is amiss
It could be the small opening of the pressure control valve that let's pressure sip from buffer vessel into reactor that causes the pressure differential and maybe temperature drop(?)
Else, I'm not sure how this freezing outside pipeline occurs
Please advise,
Thank you
I would like to ask why is there ice formation on the exterior of the pipes between compressor to reactor when there is no flow passing through
or what kind of phenomenon is this?
My colleagues have suggested PV=nRT and adiabatic process as the possible reasons but I still feel that something is amiss
It could be the small opening of the pressure control valve that let's pressure sip from buffer vessel into reactor that causes the pressure differential and maybe temperature drop(?)
Else, I'm not sure how this freezing outside pipeline occurs
Please advise,
Thank you