- #1
prakhargupta3301
- 58
- 1
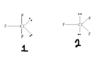
I know ClF3 has triagonal bi-pyramidal arrangement and T-shape molecular geometry. (as shown in first diag.). However, it can also be 2nd case. In this one, the shape will be triagonal planar. Also, electrons will be farthest. So why isn't ClF3 like second case?