- #1
kokodile
- 23
- 0
Member advised to choose thread titles that are descriptive of the problem.
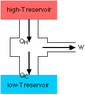
I had an exam last week and I just got it back today. On the exam was a question that I got wrong even though his wording was terrible (he's from India) and I feel that it was not clearly expressed what he was saying. The question is: "A heat engine is 20% efficient. If it absorbs 500 J of heat from reservoir. What is the work done by engine and heat rejected to the cold reservoir?"
I would like to see what answers you all come up with
Relevant equations
W.D=Qh-Qc
Efficiency=1-(Qc/Qh)
I would like to see what answers you all come up with
Relevant equations
W.D=Qh-Qc
Efficiency=1-(Qc/Qh)