anhnha
- 179
- 1
Hi,
Can anyone help me? What is the difference in mechanisms in which IB1 and IB2 are formed in the picture below?
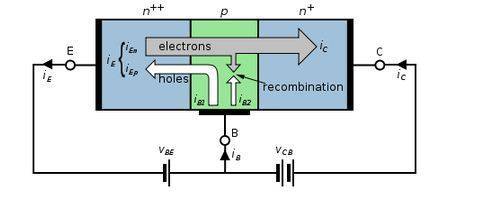
http://en.wikipedia.org/wiki/File:NPN_BJT_Basic_Operation_(Active).svg
Thanks.
Can anyone help me? What is the difference in mechanisms in which IB1 and IB2 are formed in the picture below?
http://en.wikipedia.org/wiki/File:NPN_BJT_Basic_Operation_(Active).svg
Thanks.
