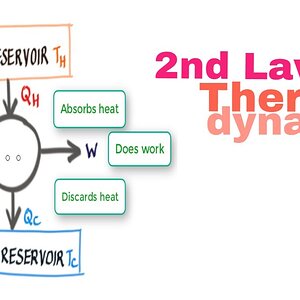
Second law of thermodyanmics Definition and 25 Threads
-
C
Work greater than heat given to colder reservoir -- impossible?
photo below... Is it possible to make a machine that would take more heat from the hot reservoir to do work than what would hot reservoir give to the cold reservoir(heat)? Apparently, it's impossible because it violates 1st law of thermodynamics. the thickness of the arrows symbolizes the...- Callmelucky
- Thread
- First law of thermodynamics Heat Impossible Second law of thermodyanmics Work
- Replies: 18
- Forum: Introductory Physics Homework Help
-

High School Question regarding Heat Transfer in Carnot Engine
***A Carnot Engine*** is a theoretical engine unlike a Sterling Engine which can be made practically. Some of the drawbacks of Carnot Engine are, 1)The Heat Transfer occurs only during isothermal process(compression and expansion),this is because the working material (ie) gas or fuel used, if...- Harikesh_33
- Thread
- Carnot Carnot engine Engine Heat Heat transfer Second law of thermodyanmics Thermal dynamics
- Replies: 1
- Forum: Thermodynamics
-
S
Undergrad Is Entropy in the Universe Lower Now Than After the Big Bang Due to Heat?
If the universe was very hot right after the Big Bang how come the entropy of the universe was lower at that point than now? Isn't heat a reason for higher entropy? -
K
Undergrad Theoretical maximum efficiency of a heat engine without Carnot
Through an intriguing fictitious dialog between Sadi Carnot and Robert Sterling, Prof. Israel Urieli of the Ohio University shows that it is not required to invoke entropy, the second law of thermodynamics, and the Carnot cycle with the [ideal] adiabatic processes in order to find out the...- KedarMhaswade
- Thread
- Carnot Carnot cycle Efficiency Engine Entropy Heat Heat engine Maximum Second law of thermodyanmics Theoretical Thermodaynamics
- Replies: 1
- Forum: Thermodynamics
-
Second Law of Thermodynamics and Heat Engines #11
The Second Law of Thermodynamics is not an easy topic. However, if you understand the concept of direction of thermodynamic processes and heat engines, you w...- Vish
- Media item
- heat second law of thermodyanmics thermodynamics
- Comments: 0
- Category: Thermodynamics
-
E
Engineering Efficiency of Heat Engines & Refrigerators: Is Impossible Possible?
For the heat engine: First I converted all the temperatures to Kelvin, ηmax=1-(333)/(1000)=0.667 ηclaim=(1*10^3)/(1.75*10^3)=0.5714 So the heat engine seems to be less efficient than a Carnot heat engine which means it can exist. For the refrigerator: COPmax=(253)/(363-253)=2.3...- EngineeringStudent
- Thread
- Efficiency Engines Heat Heat engines Impossible Refrigerators Second law of thermodyanmics Thermodaynamics
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-

Undergrad The second law of thermodynamics -- What does "from cold to hot" mean?
in Clausius formulation, what does the phrase "from cold to hot" means? I can understand it intuitively but in the language of the zero and first laws, we have not defined a temperature scale, only equivalence classes of systems that will be in equilibrium with each other (systems with the same...- QuasarBoy543298
- Thread
- Cold Hot Law Mean Second law Second law of thermodyanmics Thermodynamics
- Replies: 8
- Forum: Thermodynamics
-
E
Graduate Why does the entropy of the Universe always increase?
i don't really understand why S of the universe must be always positive,i know that only reversible process have constant entropy but why real proceses always increase S in the universe? sorry for bad english I am not from USA or UK- Est120
- Thread
- Entropy increase Second law of thermodyanmics Thermodaynamics Universe
- Replies: 4
- Forum: Thermodynamics
-
F
Graduate Machine in Clausius' 2nd law of thermodynamics?
Hi all, sorry for the condensed title of my post. Any other version of the question I'm trying to ask turned out to be longer than allowed. So, my question is about the wording in some versions of Clausius' statement of the 2nd law of thermodynamics. From time to time I read something like...- FranzDiCoccio
- Thread
- 2nd law Clausius Law Machine Second law of thermodyanmics Thermodynamics
- Replies: 7
- Forum: Thermodynamics
-
T
Graduate Entropy in the Brazil Nut Sorting Effect
In Brazil Nut effect /Granular convection the large grains move upward and the smaller ones go downward. This sorting is supposed to reduce the multiplicity of this system. But according to the second law of thermodynamics, entropy and multiplicity of the system should increase. I am looking...- Tahmeed
- Thread
- Brazil Convection Entropy Granular Second law of thermodyanmics Sorting
- Replies: 4
- Forum: Thermodynamics
-
R
Undergrad Heat death of the universe and the 3rd law of thermodynamics
If the universe keeps expanding and eventually ends in a "big freeze" or heat death, does this contradict the third law of thermodynamics? The third law of thermodynamics states that a crystal at absolute zero has zero entropy. Since the entropy of the universe can never decrease, as the age...- Robert Leslie
- Thread
- Absolute zero Death Entropy Heat Heat death Law Second law of thermodyanmics Thermodynamics Universe
- Replies: 4
- Forum: Cosmology
-
D
Van der Waal expansion and delivered work
Homework Statement Assume that one mole of an ideal van der Waals fluid is expanded isothermally, at temperature T_h from an initial volume V_i to a final volume V_f. A thermal reselvoir at temperature T_c is available. Apply dW_{RWS} = \left ( 1 - \frac{T_{RHS}}{T} \right ) (-dQ) +(-dW) to a...- Dazed&Confused
- Thread
- Expansion Maximum work Second law of thermodyanmics Thermodynamics Work
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Undergrad Does Building Construction Defy the Second Law of Thermodynamics?
From what I know, the law says that disorder increases over time. But, when a building is constructed the disordered bricks,cement etc. take form of the ordered building. Am I wrong or is this an exception?- shihab-kol
- Thread
- Disorder Law Second law Second law of thermodyanmics Thermodynamcics Thermodynamics
- Replies: 10
- Forum: Thermodynamics
-
M
Graduate Doubts Arising from Clausius' Inequality and the Second Law
I began reading Mehran Kardar's Statistical Physics of Particles and about halfway through the first chapter, there was a discussion on the second law of thermodynamics. He makes no mention of the old tenet that 'the total entropy in the universe must always increase' (I'll refer to this as the...- modulus
- Thread
- Clausius Doubts Entropy Inequality Law Second law Second law of thermodyanmics
- Replies: 6
- Forum: Thermodynamics
-

Determine the coefficient of performance of this cycle
Homework Statement In an ideal refrigeration cycle, the temperature of the condensing vapour is 40oC and the temperature during evaporation is -20oC. Determine the coefficient of performance of this cycle for the working fluids; R12 and ammonia. Homework Equations C.O.Pc = TL/(TH-TL) The...- Nemo's
- Thread
- Carnot refrigerator Coefficient Cycle performance Second law of thermodyanmics Thermodynamcics Thermodynamics second law
- Replies: 4
- Forum: Engineering and Comp Sci Homework Help
-
V
Doubt from second law of thermodynamics
qrev/T = ΔS here what does ΔS signify?does it mean change in entropy of system or surroundings? how is entropy of system,surrounding and universe related to each other and which entropy is used in gibbs free energy equation?- vijayramakrishnan
- Thread
- Doubt Entropy Law Second law Second law of thermodyanmics Thermodynamcics Thermodynamics
- Replies: 1
- Forum: Chemistry
-

Undergrad Doubt regarding proof of Clausius Inequality.
I have attached two images from my textbook one of which is a diagram and the other a paragraph with which I am having problems. The last sentence mentions that due to violation of 2nd law we cannot convert all the heat to work in this thermodynamic cycle. However what is preventing the carnot...- weezy
- Thread
- Clausius Doubt Entropy Inequality Proof Second law of thermodyanmics Thermodyamics
- Replies: 5
- Forum: Thermodynamics
-
Z
Thermodynamics: Irreversible process and entropy
Homework Statement Hi ! I'm stuck with these two questions of my assignment of thermodynamics - Give two exemples of irreversible process (initial state, process, final state) - For each of them, explain why they are irreversible on the microscopic scale. Homework Equations We are not asked...- zanthia
- Thread
- Entropy Irreversible Process Second law of thermodyanmics Thermodynamcics Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
O
Graduate Using a hot gas to drive a piston: entropy reduction?
Suppose we have an insulated cylindrical container with a piston inserted from one end. Suppose the volume confined by the piston is full of a hot gas. Now let the gas drive the piston so that the volume is increased. Did the entropy of the system decrease because some of the energy of the...- omoplata
- Thread
- Cylinder Drive Entropy Gas Hot Piston Reduction Second law of thermodyanmics
- Replies: 1
- Forum: Thermodynamics
-

Graduate Does the atmosphere cool with altitude due to gravity?
I read an article a day or so ago titled Hydrostatic Lapse, which makes the case for a phenomenon that I thought was well and truly confirmed; that gravity is responsible for the cooling of air with altitude. However I discover in the sequel article The Gemini Cycle that this phenomenon is...- kyle Bacon
- Thread
- Altitude Atmosphere Cool Gravity Second law of thermodyanmics Thermodyamics
- Replies: 63
- Forum: Thermodynamics
-

Entropy change of a reservoir after heating something up
Homework Statement 1kg of silver is heated by a large heat reservoir at 373 K from 273K. Calculate the change of entropy in: a) the silver b) the reservoir c) the universe. Homework Equations ΔS = ∫dQ/T The Attempt at a Solution calculating the change in the silver first ΔS = ∫dQ/T...- Robsta
- Thread
- Change Entropy Heating Second law of thermodyanmics Thermodynamics
- Replies: 3
- Forum: Introductory Physics Homework Help
-
R
Graduate Adiabatic irreversible expansion
This example is taken from the wikipedia page describing irreversible processes. I just want to make sure I understand correctly why the initial state can't be reached anymore. I assume the transitions to be quasi-static, but there is friction between the piston and the cylinder. If so...- Razvan
- Thread
- Adiabatic Adiabatic expansion Expansion Irreversible Second law of thermodyanmics Thermodynamics
- Replies: 25
- Forum: Thermodynamics
-
2
Undergrad Confused about the Carnot engine?
I am teaching myself thermodynamics (and really enjoying it!) but am slightly confused about Carnot's engine. From the equation efficiency=1-T(cold reservoir)/T(hot reservoir), I see that the most efficient engine is one where the difference in temperature between the cold and hot reservoirs is...- 21joanna12
- Thread
- Carnot Carnot cycle Carnot engine Confused Engine Heat and thermodynamics Second law of thermodyanmics Thermodynamics
- Replies: 4
- Forum: Thermodynamics
-
Graduate Probabilistic particle-scale valve with no moving parts
Hi, Smoluchowski's trap door was proven to fail to obtaining work in a system without heat difference. The problem turns is random movement of trap door due to random particle movements. However I could not find any discussions of such system where the "trap door" did not involve moving parts...- Filip Kierzek
- Thread
- parts Second law of thermodyanmics Valve
- Replies: 5
- Forum: High Energy, Nuclear, Particle Physics
-

Graduate Universe : Can time move backward (New theory special)
So, Hey Guys, I was watching DNews and Suddenly I came across this video. Also I found two sources of 'theory' Source A ; Source B; Seems like they are saying Entropy isn't the factor Explaining time, They even said that Thermodynamics (and it's second law) isn't what explains the time, They...- CaptCoonoor
- Thread
- Gravity Relativity Second law of thermodyanmics Theory Thermodynamics Time Universe
- Replies: 4
- Forum: Beyond the Standard Models