- #1
harimakenji
- 94
- 0
about "matter"
1. The forces between molecules in a substance are primarily
a. electrical
b. gravitational
c. chemical
d. mechanical
2. If a substance is heated
a. the molecules lose potential energy
b. the molecules lose kinetic energy
c. the temperature determines the quantity of internal energy
d. the internal energy of the substance is increased
3. Which one is right if the liquid is mercury
a.
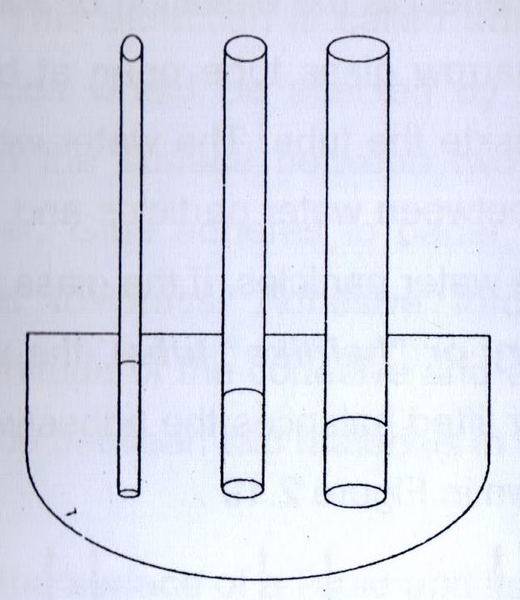
b. http://wikipremed.com/image_science_archive_th/010108_th/117850_256px-CapillaryAction.svg_68.jpg (only observe first two tubes from right)
1. answer : a
reason : not sure, just read it somewhere and don't know why. Can someone please explain it why?
2. between (a) and (d), but I chose (d).
reason : if the substance is heated, it will absorbs heat energy and internal energy increases?
3. the difference between first and second picture is the height of mercury. In picture (a), the smaller the tube, the mercury will rise closer to to the surface. In picture (b), the smaller the tube (the right-most tube), the mercury will drop further from the surface.
My answer : second picture is correct because the smaller the tube, cohesion will be bigger and mercury molecules will attract each other more and cause the it to drop further.
Please help me. Thank you very much
Homework Statement
1. The forces between molecules in a substance are primarily
a. electrical
b. gravitational
c. chemical
d. mechanical
2. If a substance is heated
a. the molecules lose potential energy
b. the molecules lose kinetic energy
c. the temperature determines the quantity of internal energy
d. the internal energy of the substance is increased
3. Which one is right if the liquid is mercury
a.
b. http://wikipremed.com/image_science_archive_th/010108_th/117850_256px-CapillaryAction.svg_68.jpg (only observe first two tubes from right)
Homework Equations
The Attempt at a Solution
1. answer : a
reason : not sure, just read it somewhere and don't know why. Can someone please explain it why?
2. between (a) and (d), but I chose (d).
reason : if the substance is heated, it will absorbs heat energy and internal energy increases?
3. the difference between first and second picture is the height of mercury. In picture (a), the smaller the tube, the mercury will rise closer to to the surface. In picture (b), the smaller the tube (the right-most tube), the mercury will drop further from the surface.
My answer : second picture is correct because the smaller the tube, cohesion will be bigger and mercury molecules will attract each other more and cause the it to drop further.
Please help me. Thank you very much