Discussion Overview
The discussion revolves around the concept of the orbital dipole moment, particularly in relation to lone pairs in chemical bonding. Participants explore the conventions used in chemistry and physics regarding dipole moments and their directional indicators.
Discussion Character
- Debate/contested
- Conceptual clarification
Main Points Raised
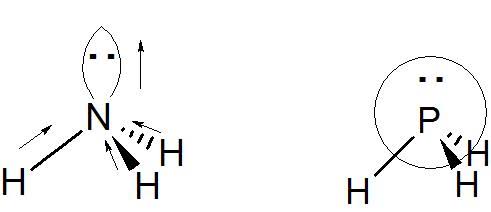
- Some participants note that the dipole moment due to lone pairs is directed from the central atom towards the end of the hybridized orbital.
- Others highlight the difference in conventions between chemistry and physics regarding the direction of dipole moments, with chemistry indicating direction from δ+ to δ- and physics pointing towards positive charges.
- A participant questions why the dipole moment of the lone pair is directed upwards from the central atom to the extremity of the hybridized lone pair orbital.
- Another participant expresses skepticism about the existence of a convention that indicates dipole moments, suggesting that arrows denote bond polarity instead.
- One participant references a specific learning resource to illustrate their understanding of dipole moment representation.
- A later reply suggests that the differences in understanding may be country-specific.
Areas of Agreement / Disagreement
Participants do not reach a consensus on the conventions for representing dipole moments, with multiple competing views and interpretations presented throughout the discussion.
Contextual Notes
The discussion reflects varying interpretations of dipole moment conventions and their application to lone pairs, with no resolution on the definitions or representations used.