Discussion Overview
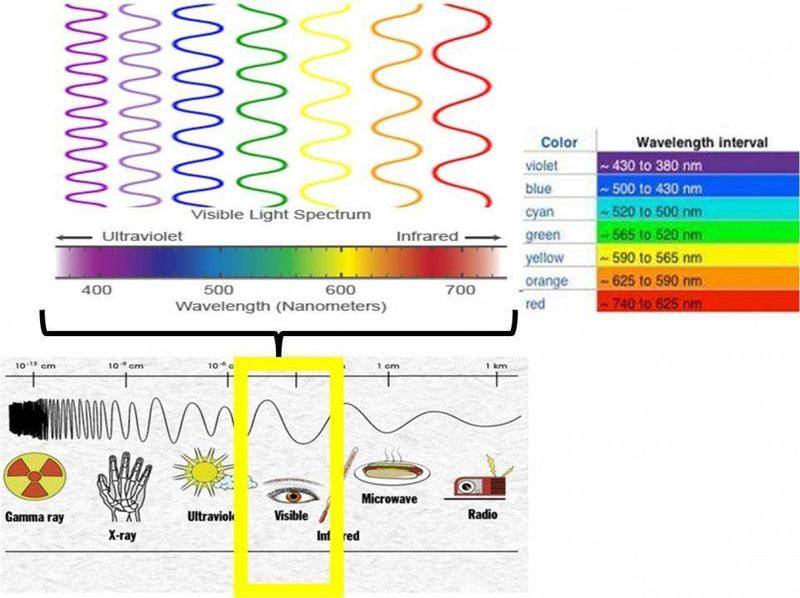
The discussion revolves around the nature of single photons and their relationship to color perception, particularly in the context of white light and the visible spectrum. Participants explore the concept of color as it relates to photons, questioning the minimum number of photons required to perceive color and the wavelengths associated with single photons.
Discussion Character
- Exploratory
- Debate/contested
- Conceptual clarification
Main Points Raised
- Some participants suggest that color is a human perception and that there is no universal consensus on color boundaries, advocating for the use of frequency as a more precise measure.
- Others argue that the spectrum is continuous and that classifications like "green" and "yellow" are arbitrary, with no physical relevance to the concept of "base photons."
- It is proposed that while a single photon can have any wavelength, it cannot be perceived as a color due to the limitations of human vision.
- Some participants note that a collection of photons is necessary to stimulate the color-sensitive receptors in the eye sufficiently to produce a perception of color.
- There is a discussion about the nature of light, with some emphasizing that light is typically a mixture of frequencies rather than a single frequency.
- One participant mentions that while a single photon does not produce a perception of color, it can have any frequency, thus addressing the question of what frequency a single photon can possess.
- There is a suggestion that the perception of color is influenced by the proportions of different wavelengths that stimulate the receptors in the eye.
Areas of Agreement / Disagreement
Participants generally agree that a single photon cannot be perceived as a color, but there is disagreement on the implications of this regarding the nature of photons and color perception. Multiple competing views remain on how to best describe the relationship between frequency, wavelength, and color perception.
Contextual Notes
Limitations include the dependence on human perception and the arbitrary nature of color classifications. The discussion also highlights the complexity of light as a mixture of frequencies and the challenges in defining color in physical terms.
Who May Find This Useful
This discussion may be of interest to those studying optics, color theory, or the physics of light, as well as individuals involved in applications related to visual perception and design.