- #1
pac0master
- 13
- 1
[Question]
So I was thinking about Physics for some time and for the sake of
curiosity I've came with this problem:
Let's say we have a liquid flowing into a system with infinite
space.
The flow is constant ( F )
The liquid decays over time with a half life ( λ )
We're looking for the Total mass (or volume (M') left in the system
after a certain amount of time ( T )
Example:
If a liquid with a Half life of 5 Days flows in a container at a
rate of 250'000L per days, How much will we have left after 27
Days
[Difficulty]
I've been told that I need Calculus, Something like Integral,
Unfortunately I never learned it.
This is also simply a question from Curiosity, There is no plan in my near future to learn or use Calculus. meaning that to solve it myself, I would need to works months or Years learning a new type of mathematics.
I'm just curious to know how to solve this.
It was inspired by the Fukushima disaster where some nuclear contamination is dumped into the Ocean
Of course it's oversimplified.
[Thoughts]
I could calculate it if it was a Closed system, (where no matter
enters or leave the system(other than the decay))
Here I did an image with the formula I've "created" (was bored at school)
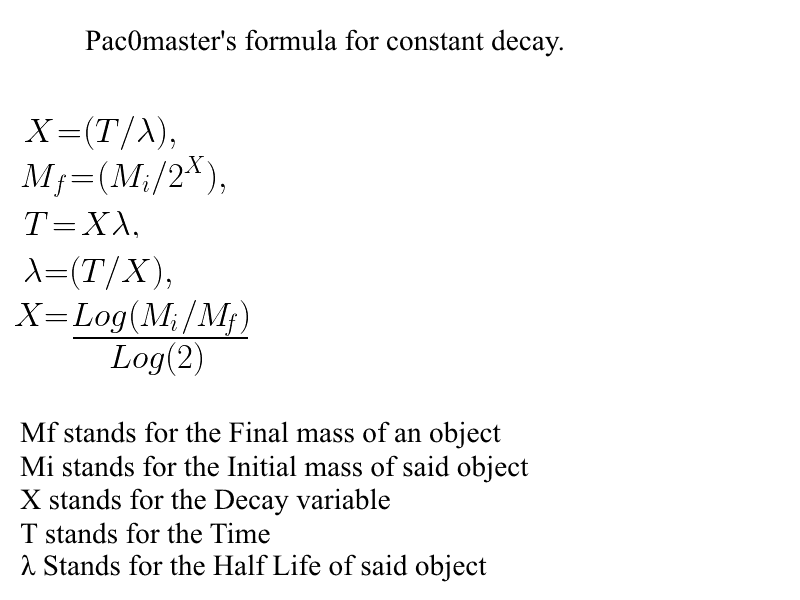
It's basic but it works. I've also made it into an Excel Sheet which is pretty cool.
So it rise the question. Is it possible to solve my problem in Excel as well, or perhaps softwares like Wolfram Alpha?
But just like I've stated above, It only works for closed system, where we start with an initial amount and let the decay do it job.
It's a simplified version of the C14 Dating method..Thanks for the help
So I was thinking about Physics for some time and for the sake of
curiosity I've came with this problem:
Let's say we have a liquid flowing into a system with infinite
space.
The flow is constant ( F )
The liquid decays over time with a half life ( λ )
We're looking for the Total mass (or volume (M') left in the system
after a certain amount of time ( T )
Example:
If a liquid with a Half life of 5 Days flows in a container at a
rate of 250'000L per days, How much will we have left after 27
Days
[Difficulty]
I've been told that I need Calculus, Something like Integral,
Unfortunately I never learned it.
This is also simply a question from Curiosity, There is no plan in my near future to learn or use Calculus. meaning that to solve it myself, I would need to works months or Years learning a new type of mathematics.
I'm just curious to know how to solve this.
It was inspired by the Fukushima disaster where some nuclear contamination is dumped into the Ocean
Of course it's oversimplified.
[Thoughts]
I could calculate it if it was a Closed system, (where no matter
enters or leave the system(other than the decay))
Here I did an image with the formula I've "created" (was bored at school)
It's basic but it works. I've also made it into an Excel Sheet which is pretty cool.
So it rise the question. Is it possible to solve my problem in Excel as well, or perhaps softwares like Wolfram Alpha?
But just like I've stated above, It only works for closed system, where we start with an initial amount and let the decay do it job.
It's a simplified version of the C14 Dating method..Thanks for the help
Last edited: