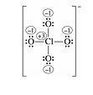
SUMMARY
The discussion focuses on drawing multiple Lewis dot structures for the chlorate ion (ClO4-). Participants emphasize the importance of calculating formal charges to determine the most favorable structure. Suggestions include using double bonds to minimize charge separation and acknowledging that some compounds may violate the octet rule for stability. The conversation references Linus Pauling's contribution to understanding mesomeric states through the concept of "dotted chemical bonds."
PREREQUISITES
- Understanding of Lewis dot structures
- Knowledge of formal charge calculation
- Familiarity with octet rule exceptions
- Basic concepts of chemical bonding and resonance
NEXT STEPS
- Research methods for calculating formal charges in polyatomic ions
- Explore resonance structures and their significance in molecular stability
- Learn about exceptions to the octet rule in various chemical compounds
- Investigate Linus Pauling's theories on chemical bonding and resonance
USEFUL FOR
Chemistry students, educators, and professionals involved in molecular structure analysis and chemical bonding concepts.