kotreny
- 46
- 0
Hey everyone, I just thought of a mind-boggling "thought experiment":
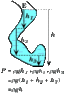
As a reminder, the formula for water pressure acting on an object due to weight is:
(Water density)*(Acceleration of gravity)*(Depth of object underwater)
Note that the shape of the container doesn't matter.
Imagine a water-filled tank the size of the Pacific Ocean (exact measurements won't be necessary). The tank is completely filled with water and is sealed shut, so any pressure other than water pressure is absent. Now imagine that at one corner of the tank there is an incredibly narrow "chimney" about ten nanometers in diameter and extending about ten kilometers above the top of the tank. This tube is connected directly to the big tank and is sealed shut, though the water level can be changed at will. I didn't do the math, but I'm pretty sure a few drops of water would raise the water level by at least hundreds of meters.
The weird part: According to the formula, water pressure throughout a container is dependent only upon the height of the container, assuming gravity and density remain constant. Technically, our imaginary tank is over ten kilometers tall, since the chimney is part of the tank. Does this mean that adding just a few drops of water can create enormous pressure everywhere in the ocean-sized tank? Seems to violate the law of conservation of energy, doesn't it?
Yes, I know that pressure is force/area, so the weight of the drops is concentrated like a needle. But can anyone tell me how--preferably on a molecular level--that force is distributed to every part of a body of water as big as the PACIFIC OCEAN?
Thanks
As a reminder, the formula for water pressure acting on an object due to weight is:
(Water density)*(Acceleration of gravity)*(Depth of object underwater)
Note that the shape of the container doesn't matter.
Imagine a water-filled tank the size of the Pacific Ocean (exact measurements won't be necessary). The tank is completely filled with water and is sealed shut, so any pressure other than water pressure is absent. Now imagine that at one corner of the tank there is an incredibly narrow "chimney" about ten nanometers in diameter and extending about ten kilometers above the top of the tank. This tube is connected directly to the big tank and is sealed shut, though the water level can be changed at will. I didn't do the math, but I'm pretty sure a few drops of water would raise the water level by at least hundreds of meters.
The weird part: According to the formula, water pressure throughout a container is dependent only upon the height of the container, assuming gravity and density remain constant. Technically, our imaginary tank is over ten kilometers tall, since the chimney is part of the tank. Does this mean that adding just a few drops of water can create enormous pressure everywhere in the ocean-sized tank? Seems to violate the law of conservation of energy, doesn't it?
Yes, I know that pressure is force/area, so the weight of the drops is concentrated like a needle. But can anyone tell me how--preferably on a molecular level--that force is distributed to every part of a body of water as big as the PACIFIC OCEAN?
Thanks