Vishesh Jain
- 9
- 0
Please post this type of questions in the HW section using the template and showing your work.
Hey folks
ZnS has a ccp structure with S2- ions forming FCC lattice with Zn2+ ions at half the tetrahedral voids
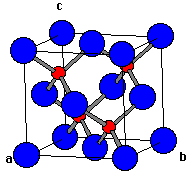
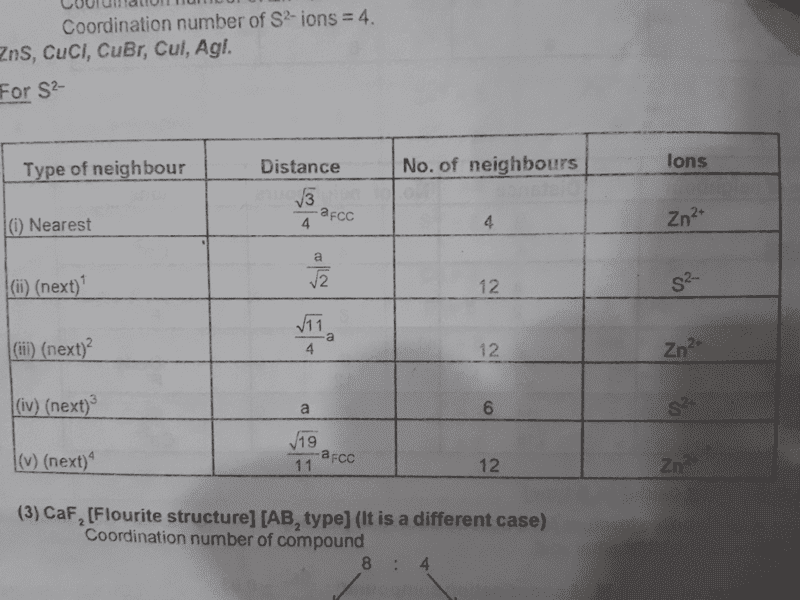
Attached is a table showing no. of nearest neighbours and their distance for an S2- ion
'a' is edge length of the unit cell
I want to know how the distance to the 3rd & 5th nearest ion is √11a/4 & √19a/11, i.e how to prove this.
 Thanks ... any help is appreciated
Thanks ... any help is appreciated
ZnS has a ccp structure with S2- ions forming FCC lattice with Zn2+ ions at half the tetrahedral voids
Attached is a table showing no. of nearest neighbours and their distance for an S2- ion
'a' is edge length of the unit cell
I want to know how the distance to the 3rd & 5th nearest ion is √11a/4 & √19a/11, i.e how to prove this.