- #1
sandmanvgc
- 26
- 1
- Homework Statement
- If the question was asking for (dry) volume, how would you do that?
- Relevant Equations
- In photos below
If the question was asking for (dry) volume, how would you do that?
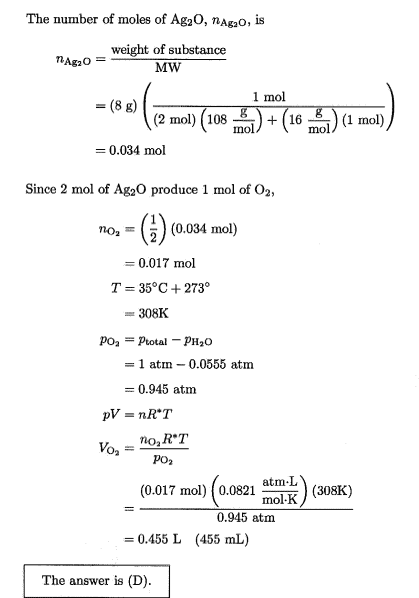
Why wouldn’t you subtract 0.555 due to water vapw from there?Lnewqban said:With no vapor of water present, pressure of oxygen would be 1 atm.
I’m not sure what to take from this? I see ptot is sum all gases. For taking dry volume, why wouldn’t you subtract p due to vapor h2o?Lnewqban said:Please, see:
https://en.m.wikipedia.org/wiki/Dalton's_law
sandmanvgc said:Why wouldn’t you subtract 0.555 due to water vapw from there?
Why don't you subtract the pressure due to water vapor when calculating the dry volume?Borek said:That's what they did, no? They calculated volume of the gas collected using partial pressure of the oxygen (so the volume calculated is the wet gas volume, as water @ 0.0555 atm occupies exactly the same volume, just increasing the total pressure). Dry volume would be closer to C.
Sadly, looks like whoever wrote the calculations down made a classical beginner's mistake and rounded down all intermediate numbers, so the errors accumulate and answers given are difficult to reproduce. Dry volume should be 436 mL, wet volume should be 462 mL. That's assuming 8.00 g of Ag2O, as technically 8 g has only one significant digit.
sandmanvgc said:Why don't you subtract the pressure due to water vapor when calculating the dry volume?
In the question they solve for (Wet) VolumeBorek said:You are asking the same question for the second time. They DID subtract. Total pressure is 1 atm, dry oxygen pressure is 0.945 atm
Dry volume should be 436 mL, wet volume should be 462 mL
The ideal gas law is a mathematical equation that describes the relationship between the pressure, volume, temperature, and number of moles of an ideal gas. It is expressed as PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
To calculate the volume of an ideal gas using the ideal gas law, you need to rearrange the equation to solve for V. This can be done by dividing both sides of the equation by P, giving you V = nRT/P. Then, you can plug in the values for n, R, T, and P to solve for V.
Dry volume refers to the volume of a gas without taking into account the water vapor present in the gas. On the other hand, the volume of an ideal gas takes into account the water vapor present in the gas. This is important because water vapor can affect the pressure and volume of a gas, and therefore, the ideal gas law calculation.
The ideal gas law equation requires that pressure be measured in atmospheres (atm), volume in liters (L), temperature in Kelvin (K), and the gas constant (R) in units of L*atm/mol*K. It is important to make sure all units are consistent when plugging in values for the ideal gas law calculation.
The ideal gas law is most accurate for ideal gases, which are gases that follow the kinetic theory of gases and have no intermolecular forces. However, it can also be used for real gases under certain conditions, such as low pressure and high temperature. In these cases, the ideal gas law may not be as accurate, but it can still provide a reasonable estimate.