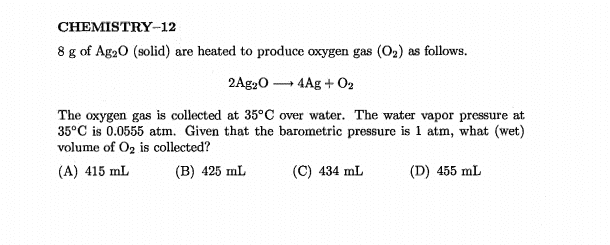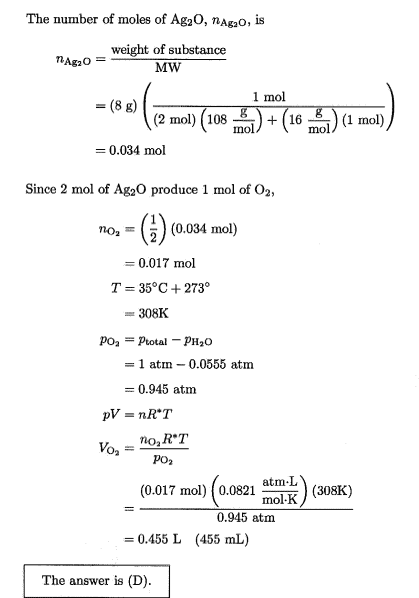Discussion Overview
The discussion revolves around calculating the (dry) volume of a gas mixture, specifically in the context of the ideal gas law, while considering the presence of water vapor. Participants explore the implications of Dalton's law and the treatment of partial pressures in determining dry versus wet volumes.
Discussion Character
- Technical explanation
- Debate/contested
- Mathematical reasoning
Main Points Raised
- Some participants question how to calculate dry volume when water vapor is present, specifically whether to subtract the pressure due to water vapor from the total pressure.
- There is a reference to Dalton's law, with participants discussing the total pressure being the sum of the partial pressures of the gases involved.
- One participant suggests that the calculated volume using the partial pressure of oxygen represents the wet gas volume, implying that dry volume should be calculated differently.
- Another participant proposes calculating the total number of moles of gas first, using the mole fraction of water to derive the total moles and subsequently the volume from the ideal gas law.
- Some participants express confusion about the treatment of water vapor pressure in calculations, particularly why it is subtracted for wet volume but not for dry volume.
- There are claims about errors in calculations due to rounding, affecting the reproducibility of results, with specific volumes suggested for dry and wet conditions.
Areas of Agreement / Disagreement
Participants do not reach a consensus on the treatment of water vapor pressure in calculating dry volume. Multiple competing views remain regarding whether and how to subtract the vapor pressure in these calculations.
Contextual Notes
Participants express uncertainty about the assumptions made in the calculations, particularly regarding the definitions of wet and dry volumes and the implications of rounding in intermediate steps.