Orion78
- 25
- 0
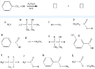
I need to find the product/s of a reaction, which has to be chosen from the list of compounds
A - L. I thought the answer was only K but it seems to be wrong or that there is another product to add.
(See the attachment). Thanks!
A - L. I thought the answer was only K but it seems to be wrong or that there is another product to add.
(See the attachment). Thanks!