guyvsdcsniper
- 264
- 37
- Homework Statement
- A reversible heat engine extracts
heat QH > 0 from a reservoir at temperature TH and heat QM = aQH > 0
from a reservoir at temperature TM ≤ TH while rejecting waste heat QC > 0
to a reservoir at temperature TC ≤ TM.
Derive an expression for the effi ciency of this three- reservoir heat
engine in terms of a and the three temperatures TH, TM, and TC ,
where the effi ciency is the total work produced divided by the total
heat extracted from the two hotter reservoirs.
- Relevant Equations
- (1 − TH /TC )/(1 + a) + (1 − TM /TC )a/(1 + a)
My book states the answer to this problem is
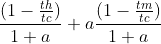 .
.
I have gotten very close to the answer. My problem is my Tc/th and tc/tm are flipped compared to the solution.
I feel like I am missing something in my algebra but can't see where I am going wrong. Could I get some help identifying where my mistake is.

I have gotten very close to the answer. My problem is my Tc/th and tc/tm are flipped compared to the solution.
I feel like I am missing something in my algebra but can't see where I am going wrong. Could I get some help identifying where my mistake is.