Titan97
Gold Member
- 450
- 18
Recently, I read two chapters from March's advanced organic chemistry. I came across gauche effect and SN2 reactions. In both phenomena, ##\sigma-\sigma^*## orbital interactions is involved. In gauche effect present in 1,2-diflouroethane, the C-F bonding orbital becomes an antibonding orbital so that C-H ##\sigma## orbital can overlap with the empty C-F ##\sigma## orbital.
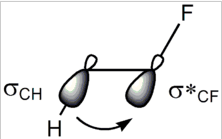
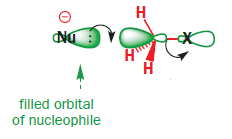
In SN2 mechanism, C-X bonding becomes antibonding orbital so that the nucleophile can attack the empty ##\sigma## orbital of Carbon
Am I correct? Is there any flaw in my understanding?
In SN2 mechanism, C-X bonding becomes antibonding orbital so that the nucleophile can attack the empty ##\sigma## orbital of Carbon
Am I correct? Is there any flaw in my understanding?