ampersand15
- 1
- 0
I'm in organic chemistry 1. We haven't gone over the Grignard reaction in class, but there was a stoichiometry problem assigned about one and I don't know how to get it started.
The following Grignard reaction is performed.
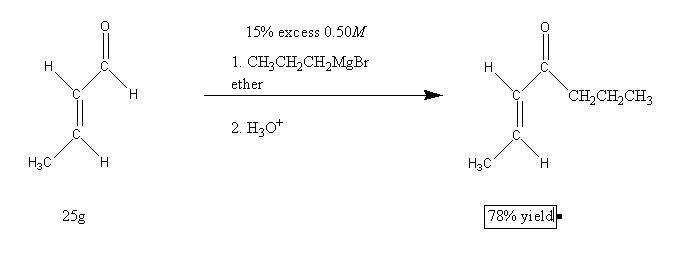
Calculate how much of a 0.50M solution of propylmagnesium bromide is required to react completely with 25g of the starting material. Assume you need a 15% excess of the Grignard reagent. Then predict the amount of product based upon a 78% yield.
If I can get past the first step it's just dimensional analysis.
I'm having trouble at the first step, balancing the reaction. I end up with 7C, 16H, 2O, 1Mg, 1Br on the reactant side, and 7C, 14H, 2O, 1Mg, and 1Br on the product side. The only thing I can do that balances the equation is to ignore the H3O+. Is this what I'm supposed to do? Not including H3O+ in the calculation leaves me with 7C, 13H, and 1O on both sides. I'm assuming this has something to do with the Grignard reaction that I haven't encountered yet?
Thanks
Homework Statement
The following Grignard reaction is performed.
Calculate how much of a 0.50M solution of propylmagnesium bromide is required to react completely with 25g of the starting material. Assume you need a 15% excess of the Grignard reagent. Then predict the amount of product based upon a 78% yield.
Homework Equations
If I can get past the first step it's just dimensional analysis.
The Attempt at a Solution
I'm having trouble at the first step, balancing the reaction. I end up with 7C, 16H, 2O, 1Mg, 1Br on the reactant side, and 7C, 14H, 2O, 1Mg, and 1Br on the product side. The only thing I can do that balances the equation is to ignore the H3O+. Is this what I'm supposed to do? Not including H3O+ in the calculation leaves me with 7C, 13H, and 1O on both sides. I'm assuming this has something to do with the Grignard reaction that I haven't encountered yet?
Thanks