- #1
fissile_uranium
- 2
- 0
- TL;DR Summary
- I'm trying to find the required target mass for a specific nuclear reaction with a fixed yield and cross-section.
I am trying to get a plot, or a result for target like this:
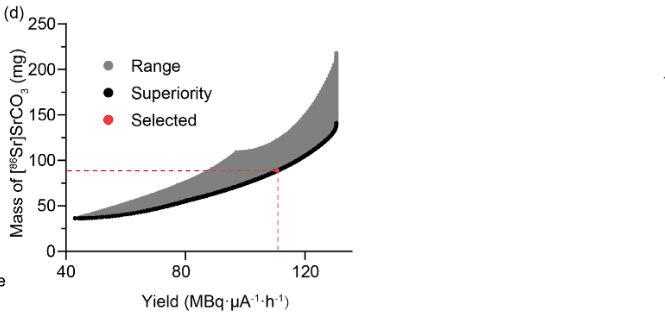
I've found some equations for my requested results, but they are from the same person's articles and I couldn't find another source. I've searched for EOB and TTY calculations but couldn't really do something. Could you give me an easy equation to apply? Also there are equations from this link:
https://www.degruyter.com/document/doi/10.1515/ract-2013-2234/html
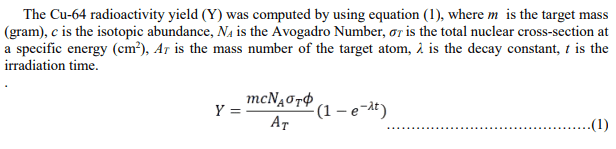
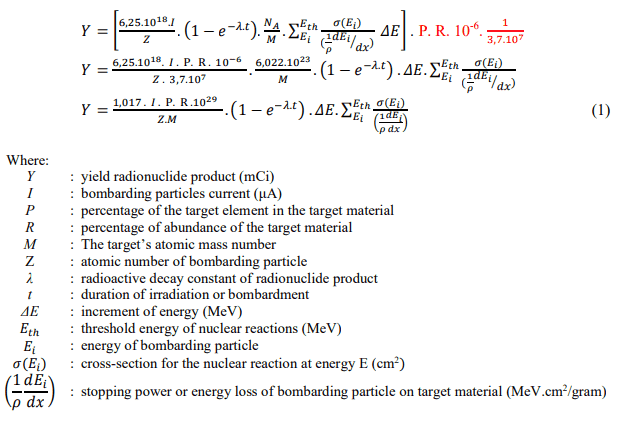
Could you help me with the wanted results? What equation should I use?
Properties:
29.4 TBq
0.05 barns
45 (target molar mass)
59.1 years (produced radionuclide half-life).
I've found some equations for my requested results, but they are from the same person's articles and I couldn't find another source. I've searched for EOB and TTY calculations but couldn't really do something. Could you give me an easy equation to apply? Also there are equations from this link:
https://www.degruyter.com/document/doi/10.1515/ract-2013-2234/html
Could you help me with the wanted results? What equation should I use?
Properties:
29.4 TBq
0.05 barns
45 (target molar mass)
59.1 years (produced radionuclide half-life).
Last edited by a moderator: