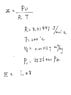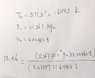EastWindBreaks
- 128
- 3
[Mentor note: Thread title changed to describe actual problem being presented]
1. Homework Statement
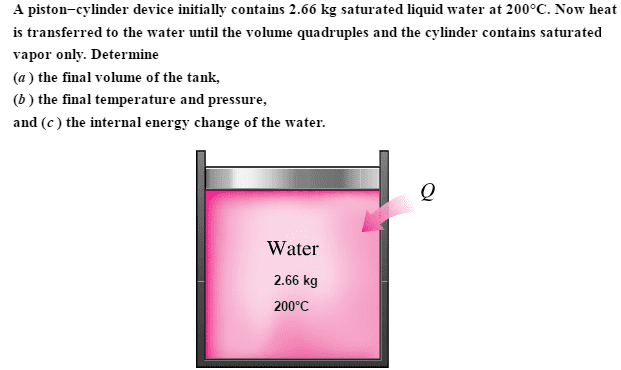
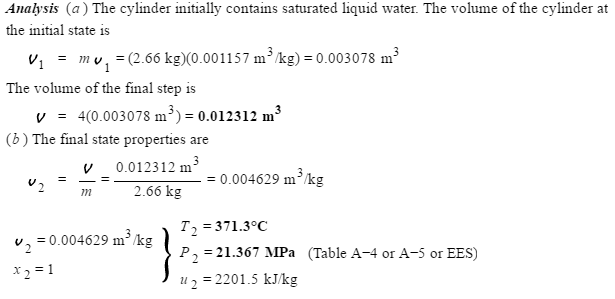
I understand you have to interpolate temperature and pressure of the saturated vapor from the table, since there is no matched final specific volume value on the saturated table.
But I don't understand why my first approach is wrong: Since the pressure is constant, I can find pressure using initial properties ( h=u+Pv, where h is the enthalpy of saturated liquid and u is the internal energy of saturated liquid) and then find T using ideal gas equation.
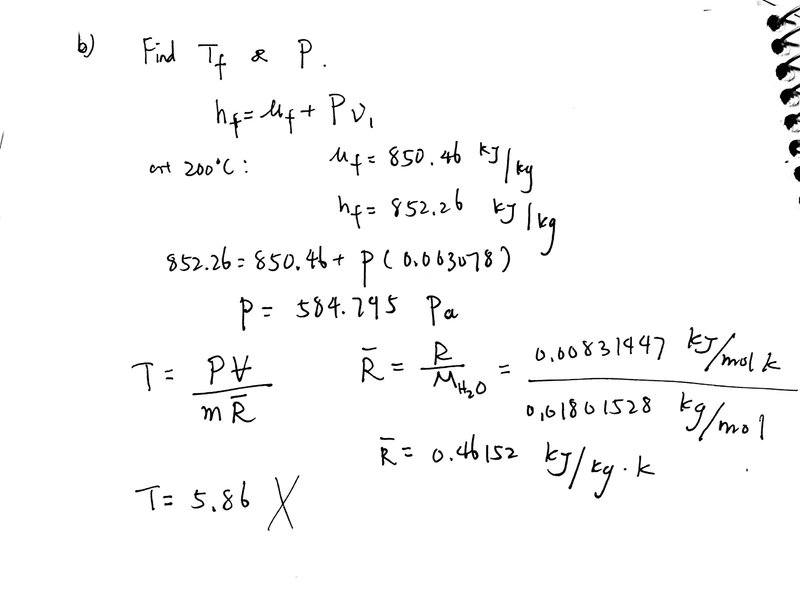 [/B]
[/B]
the pressure is not correct either, why can I not use h=u+Pv in this case?
does ideal gas equation only works near standard pressure and temperature condition?
1. Homework Statement
Homework Equations
The Attempt at a Solution
I understand you have to interpolate temperature and pressure of the saturated vapor from the table, since there is no matched final specific volume value on the saturated table.
But I don't understand why my first approach is wrong: Since the pressure is constant, I can find pressure using initial properties ( h=u+Pv, where h is the enthalpy of saturated liquid and u is the internal energy of saturated liquid) and then find T using ideal gas equation.
the pressure is not correct either, why can I not use h=u+Pv in this case?
does ideal gas equation only works near standard pressure and temperature condition?
Attachments
Last edited by a moderator: