mess1n
- 24
- 0
I'm very confused about how metal hydrides form. I'm looking at PCI plots and I see what's happening, but I have no idea why it is. For instance:
"Molecular hydrogen is dissociated at the surface before absorption; two H atoms recombine to H2 in the desorption process."
Why does this happen?
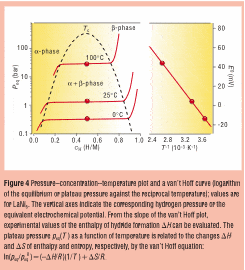
Also, I've attached a PCI plot. Can anyone explain why the concentration suddenly increases at certain pressures?
I've searched so many books and internet resources to try and find an explanation for these processes, to no avail, please help! :(
Andrew
"Molecular hydrogen is dissociated at the surface before absorption; two H atoms recombine to H2 in the desorption process."
Why does this happen?
Also, I've attached a PCI plot. Can anyone explain why the concentration suddenly increases at certain pressures?
I've searched so many books and internet resources to try and find an explanation for these processes, to no avail, please help! :(
Andrew
Last edited: