- #1
Amitayas Banerjee
- 31
- 2
So, I was studying about general properties of matter and topics like surface tension. I came across the phenomenon of water rising along a glass plate like in the picture. I looked for some mathematical interpretation of this on the internet and in some books.
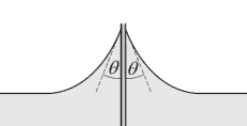
[![enter image description here][1]][1]
I looked for some mathematical interpretation of this on the internet and in some books.
I found some mathematical understanding of the phenomenon in the book **Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves** and also elaborate answers on StackExchange like this one: https://physics.stackexchange.com/q...an-water-rise-above-the-edge-of-a-glass/45122
But I decided to find the height along which the water climbs on the glass by balancing forces on the **infinitely long** water element:
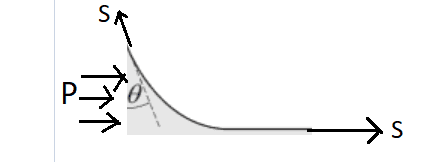
[![enter image description here][2]][2]
It is to be noted that the height of this water element is **$h$** and it has an infinite length in the horizontal direction.
Now the pressure force $P$ can be calculated as
$$P=\int_0^h \rho gz dz=\frac{1}{2}\rho g h^2 $$
On balncing forces in the horizontal direction, we get $$P+S =S\sin \theta$$ $$\Rightarrow \frac{1}{2}\rho g h^2= S(\sin \theta -1)$$ which is surely a **contradiction** as the term in the left hand side is bound to be positive. Hence I believe that I have apparently disproved the fact that water would rise along the glass plate. But I also know that this is true that water has to rise as evident from daily experiences. So, where does my math go wrong?
[1]: https://i.stack.imgur.com/FjxSP.png
[2]: https://i.stack.imgur.com/e6BNY.png
[![enter image description here][1]][1]
I looked for some mathematical interpretation of this on the internet and in some books.
I found some mathematical understanding of the phenomenon in the book **Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves** and also elaborate answers on StackExchange like this one: https://physics.stackexchange.com/q...an-water-rise-above-the-edge-of-a-glass/45122
But I decided to find the height along which the water climbs on the glass by balancing forces on the **infinitely long** water element:
[![enter image description here][2]][2]
It is to be noted that the height of this water element is **$h$** and it has an infinite length in the horizontal direction.
Now the pressure force $P$ can be calculated as
$$P=\int_0^h \rho gz dz=\frac{1}{2}\rho g h^2 $$
On balncing forces in the horizontal direction, we get $$P+S =S\sin \theta$$ $$\Rightarrow \frac{1}{2}\rho g h^2= S(\sin \theta -1)$$ which is surely a **contradiction** as the term in the left hand side is bound to be positive. Hence I believe that I have apparently disproved the fact that water would rise along the glass plate. But I also know that this is true that water has to rise as evident from daily experiences. So, where does my math go wrong?
[1]: https://i.stack.imgur.com/FjxSP.png
[2]: https://i.stack.imgur.com/e6BNY.png