Jaygo333
- 4
- 0
I've been pondering this question a while regarding how water freezes.
Assuming the temperature above a lake is 0 degrees centigrade, the water at the top of the lake would decrease in temperature until 4 degrees centigrade at which point, being at its densest, would sink towards the bottom of the lake only to be replaced with the warmer water that was sitting below it. After a time t, all the water in the lake would be 4 degrees centigrade and the water at the top would eventually freeze below 4 degrees and eventually turn to ice blocking any water underneath from freezing over.
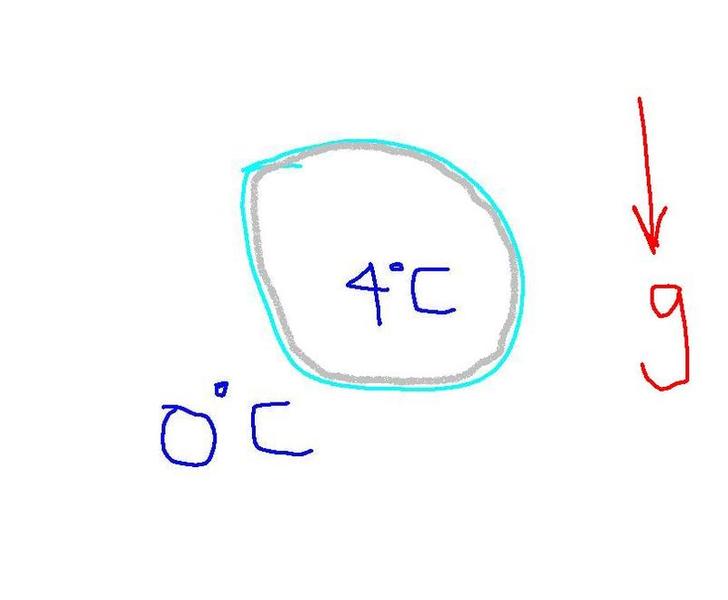
Now, knowing that to be true, assume we have a spherical ball of water suspended in mid air and the only force working on it being gravity. The temperature outside the ball is 0 degrees centigrade and the temperature inside is 4 degrees centigrade. After a time t, the ball being surrounded by freezing water on all sides, ice would form on all the sides at once, and this ice being less dense than water, will want to float.
My question being, in which way would the ice form on this ball of water? From the outside shell forming in, from the bottom up, or from the top down?
P.S. I do know that a spherical ball of water suspended in mid air is irrational thinking and that it would be better to assume the ball in a rational and more logical location such as in a vacuum, but in such a situation, the water would just freeze from the outside in.
Assuming the temperature above a lake is 0 degrees centigrade, the water at the top of the lake would decrease in temperature until 4 degrees centigrade at which point, being at its densest, would sink towards the bottom of the lake only to be replaced with the warmer water that was sitting below it. After a time t, all the water in the lake would be 4 degrees centigrade and the water at the top would eventually freeze below 4 degrees and eventually turn to ice blocking any water underneath from freezing over.
Now, knowing that to be true, assume we have a spherical ball of water suspended in mid air and the only force working on it being gravity. The temperature outside the ball is 0 degrees centigrade and the temperature inside is 4 degrees centigrade. After a time t, the ball being surrounded by freezing water on all sides, ice would form on all the sides at once, and this ice being less dense than water, will want to float.
My question being, in which way would the ice form on this ball of water? From the outside shell forming in, from the bottom up, or from the top down?
P.S. I do know that a spherical ball of water suspended in mid air is irrational thinking and that it would be better to assume the ball in a rational and more logical location such as in a vacuum, but in such a situation, the water would just freeze from the outside in.
Last edited: