Shauryafrom2006
- 3
- 0
- TL;DR Summary
- I can't figure out the derivation of this formula.
It is from [ Class 11th] SL Arora, pg no. 11.3, the heading is [11.7] Absolute Scale of Temperature.
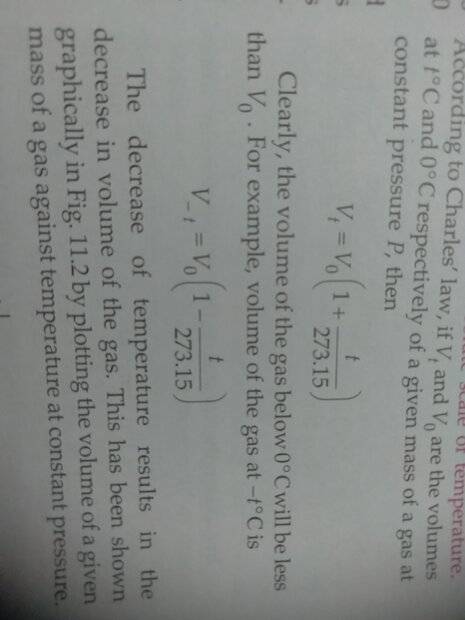
Last edited by a moderator: