- #1
mesa
Gold Member
- 695
- 38
In the Stern Gerlach experiment it would seem that an electron beam moving at some velocity bisecting the upper magnet on a horizontal path would be radially accelerated perpendicular to the direction of motion.
I am assuming from Lenz's Law this can be corrected by increasing the Bf depending on the position and direction that the electron beam is fired into the experiment, here are two scenarios:
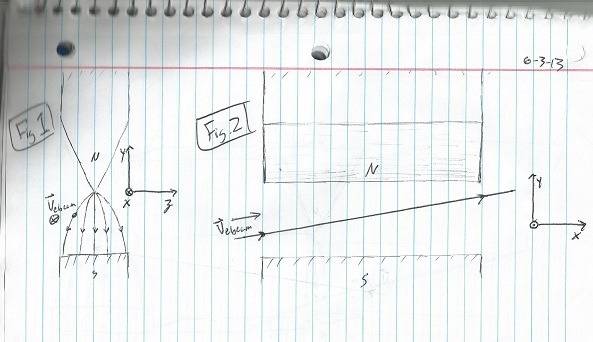
Figure 1 shows an electron beam fired horizontally along the positive x-axis slightly off center from the bisection of the experiment. As the beam is radially accelerated in the positive z direction the magnetic field increases counteracting this effect although it would seem there would still be some bending along the z axis.
Figure 2 is a representation of our beam now fired along the positive y/z plane bisecting our experiment. As the beam travels upward the Bf increases canceling the radial acceleration. This would seem like a more reasonable way to control the electron beam.
Is either of these two scenarios correct? some combination of the two? or am I just way out in left field?
I am assuming from Lenz's Law this can be corrected by increasing the Bf depending on the position and direction that the electron beam is fired into the experiment, here are two scenarios:
Figure 1 shows an electron beam fired horizontally along the positive x-axis slightly off center from the bisection of the experiment. As the beam is radially accelerated in the positive z direction the magnetic field increases counteracting this effect although it would seem there would still be some bending along the z axis.
Figure 2 is a representation of our beam now fired along the positive y/z plane bisecting our experiment. As the beam travels upward the Bf increases canceling the radial acceleration. This would seem like a more reasonable way to control the electron beam.
Is either of these two scenarios correct? some combination of the two? or am I just way out in left field?