PhysicsKush
- 29
- 4
- Homework Statement
- $$ \frac{\partial \Gamma}{\partial p} \Bigg|_{t} = \frac{\partial \Gamma}{\partial p} \Bigg|_{v} + \frac{\partial v}{\partial p}\Bigg|_{t} \frac{\partial \Gamma}{\partial v} \Bigg|_{p}$$
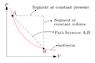
Interpret this formula by using words or a picture (ie. the change in Gamma when we pressurize a gas along an isotherm, is equal to...).
- Relevant Equations
- $$ \Gamma(p,v) = vp^2$$
$$ pv=t$$
I notice that ##pv=t## looks like the ideal gas law but with ##T## in units of energy. I know that ##pV = \text{constant}## means that the pressure of a gas decreases as you expand it (Boyle-Mariotte's law), explaining for instance how we breathe.
I guess I could put everything in words even though it will be a paragraph long and probably incorrect :
"The change in Gamma when we pressurize a gas along an isotherm is equal to the change in Gamma when we pressurize that gas along an isovolumetric process , with additionally the product between the change in volume during pressurization along an isotherm and the change in Gamma under a dynamic contrast along an isobar",
but I'm curious to see how this could be represented with a picture.
I guess I could put everything in words even though it will be a paragraph long and probably incorrect :
"The change in Gamma when we pressurize a gas along an isotherm is equal to the change in Gamma when we pressurize that gas along an isovolumetric process , with additionally the product between the change in volume during pressurization along an isotherm and the change in Gamma under a dynamic contrast along an isobar",
but I'm curious to see how this could be represented with a picture.