You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Explaining the Relation Between \gamma N_+ and 2N_{++}+N_{+-}
- Thread starter matematikuvol
- Start date
-
- Tags
- Relation
Physics news on Phys.org
1ndranil
- 16
- 1
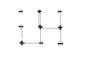
Choose a + site. Join it to each neighbor by a line. we need to draw gamma lines. Now do this for all + sites. So total lines = gamma*N(+). In this process i) between any (+,+) pair there will be two lines (one when we draw line from 1st + to 2nd +, other when we draw line from 2nd + to 1st +). Now there are N(++) number of (+,+) pair. So total lines between all (+,+) pair is 2N(++).. ii) between any (+,-) pair there will be one line (when drawn from + to -). There are N(+-) number of (+,-) pair. iii) between any (-,-) pair there will be no line. So total lines = 2N(++) + N(+-).
From the BCS theory of superconductivity is well known that the superfluid density smoothly decreases with increasing temperature. Annihilated superfluid carriers become normal and lose their momenta on lattice atoms. So if we induce a persistent supercurrent in a ring below Tc and after that slowly increase the temperature, we must observe a decrease in the actual supercurrent, because the density of electron pairs and total supercurrent momentum decrease. However, this supercurrent...
Hi. I have got question as in title. How can idea of instantaneous dipole moment for atoms like, for example hydrogen be consistent with idea of orbitals? At my level of knowledge London dispersion forces are derived taking into account Bohr model of atom. But we know today that this model is not correct. If it would be correct I understand that at each time electron is at some point at radius at some angle and there is dipole moment at this time from nucleus to electron at orbit. But how...
Similar threads
- Replies
- 10
- Views
- 1K
- Replies
- 4
- Views
- 2K
- Replies
- 0
- Views
- 793
- Replies
- 1
- Views
- 1K
- Replies
- 6
- Views
- 2K
- Replies
- 1
- Views
- 2K
- Replies
- 7
- Views
- 3K
- Replies
- 4
- Views
- 2K
- Replies
- 4
- Views
- 1K
- Replies
- 9
- Views
- 2K
Hot Threads
-
A Power spectral density of a laser's frequency noise
- Started by Malamala
- Replies: 6
- Atomic and Condensed Matter
-
A Confused about noise power spectral density measurement
- Started by Malamala
- Replies: 0
- Atomic and Condensed Matter
-
I The relationship between atoms and the Universe
- Started by bjdx
- Replies: 6
- Atomic and Condensed Matter
-
-
I The relationship between the matter, energy, time and space of atoms
- Started by bjdx
- Replies: 7
- Atomic and Condensed Matter
Recent Insights
-
Insights Why Entangled Photon-Polarization Qubits Violate Bell’s Inequality
- Started by Greg Bernhardt
- Replies: 26
- Quantum Interpretations and Foundations
-
Insights Quantum Entanglement is a Kinematic Fact, not a Dynamical Effect
- Started by Greg Bernhardt
- Replies: 11
- Quantum Physics
-
Insights What Exactly is Dirac’s Delta Function? - Insight
- Started by Greg Bernhardt
- Replies: 3
- General Math
-
Insights Relativator (Circular Slide-Rule): Simulated with Desmos - Insight
- Started by Greg Bernhardt
- Replies: 1
- Special and General Relativity
-
Insights Fixing Things Which Can Go Wrong With Complex Numbers
- Started by PAllen
- Replies: 7
- General Math
-
Insights Fermat's Last Theorem
- Started by fresh_42
- Replies: 105
- General Math