- #1
Vladimir_Kitanov
- 44
- 14
I understand everything except why do we use time between collisions instead of time of colission?
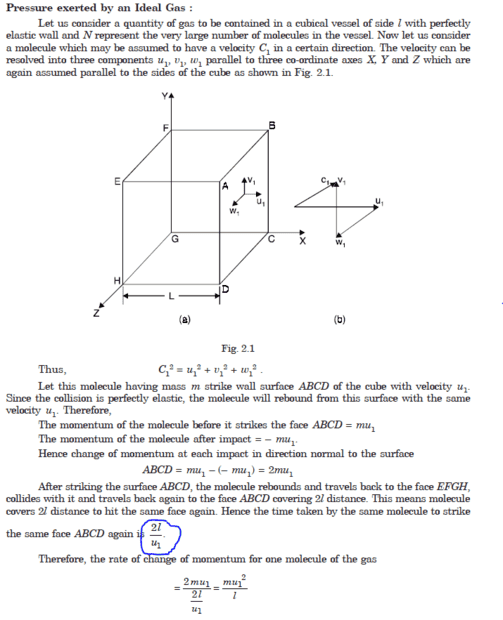
When considering the time between collisions in the kinetic equation of gases, we are looking at the average time it takes for a gas molecule to collide with another molecule in the system. This approach provides a more accurate representation of the behavior of gas particles in a given volume, as it accounts for the frequency of collisions rather than focusing on individual collision events.
Considering the time between collisions in the kinetic equation of gases allows us to better understand the distribution of speeds and energies of gas molecules in a system. By incorporating the average time between collisions, we can more accurately predict the macroscopic properties of gases, such as pressure, temperature, and volume.
The mean free path of gas molecules is directly related to the time between collisions in the kinetic equation of gases. The mean free path represents the average distance a molecule travels between collisions, which is inversely proportional to the collision frequency. By considering the time between collisions, we can calculate the mean free path and gain insights into the behavior of gas particles in a system.
Yes, accounting for the time between collisions can improve the accuracy of the kinetic theory of gases by providing a more realistic representation of gas behavior. By incorporating the average time between collisions, we can refine our predictions of gas properties and better understand the underlying principles governing the movement of gas molecules.
The time between collisions influences the pressure of a gas system by determining the frequency and intensity of collisions between gas molecules and the walls of the container. A shorter time between collisions results in more frequent and forceful impacts, leading to higher pressure. By considering the time between collisions in the kinetic equation of gases, we can accurately calculate and predict the pressure exerted by a gas in a given volume.