rloughlin03
- 3
- 0
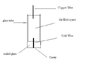
I am attempting to make a cavity microelectrode in which i will test the electro chemistry of polyaniline powder before moving on to other chemicals. I have read numerous papers on the subject. I am having a major setback in my aim: i need contact between my gold wire and the voltameter (as shown in the diagram). I originally tried mercury, but forgot that it would dissolve the gold. I am thinking of either one of three things: a graphite powder, soldering the Gold wire and copper wire together, or finding another low m.p. metal to use. I would really appreciate any ideas or feedback, especially from those who have made cavity electrodes before.
Thanks
Ryan
P.S. apologies for the crude diagram, done in a rush. Just trying to help "paint the picture"
Thanks
Ryan
P.S. apologies for the crude diagram, done in a rush. Just trying to help "paint the picture"