simon.23
- 2
- 0
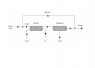
Hydrogen is used to reduce 2.3 tonne per hour, Fe2O3 to metallic iron according to the reaction
Fe_{2}O_{3} + 3H_{2} = 2Fe + 3H_{2}O
The water is condensed and the unreacted hydrogen is recycled (see diagram attached). Because the hydrogen in the fresh feed contains 1.1% CO2 as an impurity, some of the unreacted hydrogen must be purged. Calculate the flow rate and the composition of the purge stream required to limit the CO2 in the reactor feed to 2.8% if the ratio of recycle to fresh feed is 11:2 on a molar basis.
MW(Fe) = 55.85, MW(H) = 1.01, MW(O) = 16.00.
I am really struggling to get to grips with this question, ( struggling with material balance overall!)
I can do dimple material balance however for things with a recycle stream or purge stream, really confuses me.
I have the solution which I will post up, but I still don't get 'why' it is solved in this manner. I would really appreciated it, if someone explains to me why. Or if you have simpler way of working the flow rate of the purge stream?
thank you,