prehisto
- 111
- 0
Hi,Guys.
I have registered 2 spectrum's. 980nm induced spectrum of NaLaF4:Er3+ glass and NaLaF4:Er3+ ceramics.
Now, i have to explain the observed differences.
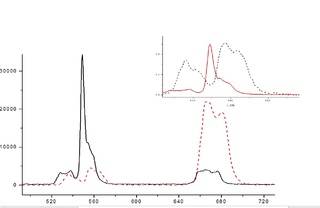
So the first one and obvious one,is that in the case of glass, luminescence bands becomes wider,because of the lattice structure of glass. Glass has more possible no-equivalent structure spots where ions can build into therefore having different electrical fields,which affect the levels of ions.
But there seemingly is another effect ,shift of the bands. Could someone can,please,explain me is it possible and if yes, why this could happen ?
There is another effect , in the case of ceramics , the intensity is much higher . I have red that that s related to phonon energy. Glass has high phonon energy,cceramics-low. But how the energy of phonos affect the intensity of luminescence is unclear for me.
p.s. this is part of my study semester ,laboratory work.
I have registered 2 spectrum's. 980nm induced spectrum of NaLaF4:Er3+ glass and NaLaF4:Er3+ ceramics.
Now, i have to explain the observed differences.
So the first one and obvious one,is that in the case of glass, luminescence bands becomes wider,because of the lattice structure of glass. Glass has more possible no-equivalent structure spots where ions can build into therefore having different electrical fields,which affect the levels of ions.
But there seemingly is another effect ,shift of the bands. Could someone can,please,explain me is it possible and if yes, why this could happen ?
There is another effect , in the case of ceramics , the intensity is much higher . I have red that that s related to phonon energy. Glass has high phonon energy,cceramics-low. But how the energy of phonos affect the intensity of luminescence is unclear for me.
p.s. this is part of my study semester ,laboratory work.
Last edited: