- #1
SpinzTronics
- 6
- 0
A friend of mine showed me this molecule and asked me about certain properties like its resonance, donating/withdrawing properties, and that was fine until he worried about the naming conventions.
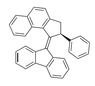
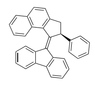
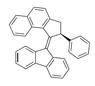
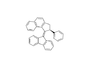
Description of molec since this site won't let me upload the pic: napthalene molec atached to 2,3 or a cyclopentane, a benzene in the 5th position, a double bond going out of cyclopentane at posotion four, and a fluorene molec attached at middle upper point of the cyclopentane in fluorene.
Honestly, looking it up was of no avail. So I tried "deconstructing" its parts:
2,3-dihydro-1H-cyclopenta[ B] napthalene or 1H-Benz(F)indene,2,3-dihydro-
5-cyclopentylbenzene
4-diene-fluorene
So would this work? 4-diene-fluorene-1H-Benz(F)indene,2,3-dihydro-5-cyclopentylbenzene
This undergrad stuff is long forgotten by me, so I would appreciate your help!
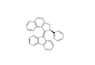
Description of molec since this site won't let me upload the pic: napthalene molec atached to 2,3 or a cyclopentane, a benzene in the 5th position, a double bond going out of cyclopentane at posotion four, and a fluorene molec attached at middle upper point of the cyclopentane in fluorene.
Honestly, looking it up was of no avail. So I tried "deconstructing" its parts:
2,3-dihydro-1H-cyclopenta[ B] napthalene or 1H-Benz(F)indene,2,3-dihydro-
5-cyclopentylbenzene
4-diene-fluorene
So would this work? 4-diene-fluorene-1H-Benz(F)indene,2,3-dihydro-5-cyclopentylbenzene
This undergrad stuff is long forgotten by me, so I would appreciate your help!
Attachments
Last edited by a moderator: