Physics lover
- 249
- 25
- Homework Statement
- Which of the following are Newman projections of meso-2,3-butanediol
- Relevant Equations
- Newman projection
Fischer projection
The question and options are-:
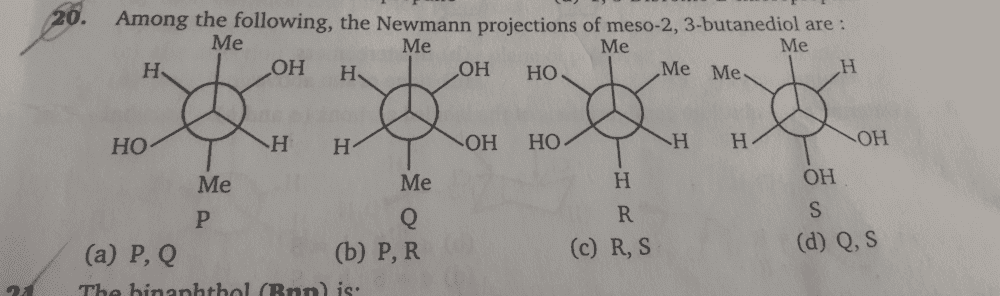
Ok so what I did was that i converted all of them to their fischer projection.But only P gave me meso-2,3-butanediol.Others were not meso.Please help.
Ok so what I did was that i converted all of them to their fischer projection.But only P gave me meso-2,3-butanediol.Others were not meso.Please help.