- #1
MexChemE
- 237
- 55
Good evening people of PF! I have recently encountered a problem from Himmelblau's Basic Principles and Calculations in Chem. E. which asks to set up an energy balance for a tank, and then non-dimensionalize the differential equation before solving it. It's not the most complex task, but it's the first time I've done it and I wanted to know if I did things right. Here's an sketch of the system.
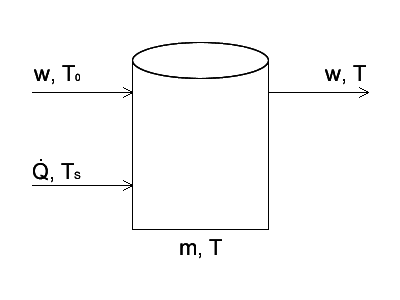
Mass flows in and out at the same rate w, therefore the mass inside the tank is a constant, m. Heat is being transferred into the contents of the tank using a coiled tube heater, in which steam flows at temperature TS. The rate of heat transfer is given by [itex]\dot{Q} = hA(T_S - T)[/itex]. Fluid entering the tank has a constant temperature of T0, which is also the temperature of the system at t = 0. The differential energy balance for the system is
[tex]\frac{\textrm{d} U}{\textrm{d} t} = \dot{Q} - w \Delta H[/tex]
In terms of temperature
[tex]m C_p \frac{\textrm{d} T}{\textrm{d} t} = hA(T_S - T) - w C_p (T-T_0)[/tex]
The problem only tells you the non-dimensional parameters you are supposed to use
[tex]\Theta = \frac{T-T_0}{T_S - T_0}[/tex]
[tex]\tau = \frac{w}{m} t[/tex]
[tex]\beta = \frac{hA}{w C_p}[/tex]
Which are temperature, time, and an additional constant parameter. In order to start scaling the equation I decided to divide the entire equation by wCp(TS-T0). Also, I've seen some heat transfer textbooks use the relation dT = d(T-T0), because T0 is a constant, so I decided to apply said relation and ended up with the following equation
[tex]\frac{m C_p}{w C_p} \frac{\textrm{d}}{\textrm{d} t} \left( \frac{T-T_0}{T_S-T_0} \right) = \frac{hA(T_S - T)}{w C_p (T_S - T_0)} - \frac{w C_p (T-T_0)}{w C_p (T_S - T_0)}[/tex]
[tex]\frac{m}{w} \frac{1}{\frac{m}{w}} \frac{\textrm{d} \Theta}{\textrm{d} \tau} = \beta \frac{T_S - T}{T_S - T_0} - \Theta[/tex]
Using
[tex]\frac{T_S - T}{T_S - T_0} = 1 - \Theta[/tex]
We get the non-dimensionalized energy balance
[tex]\frac{\textrm{d} \Theta}{\textrm{d} \tau} = \beta (1- \Theta) - \Theta[/tex]
[tex]\frac{\textrm{d} \Theta}{\textrm{d} \tau} = \beta - (\beta +1) \Theta[/tex]
In my opinion, it is not necessary to non-dimensionalize the original D.E. in order to solve it, however, I guess it's a good training problem for non-dimensionalization. I was hoping it could get some insights about how to perform the non-dimensionalization procedure more efficiently. Also, I was wondering, if one is not given the non-dimensional parameters to use, is there a set of rules for choosing them or is it just common sense and practice?
Thanks in advance for any input!
Mass flows in and out at the same rate w, therefore the mass inside the tank is a constant, m. Heat is being transferred into the contents of the tank using a coiled tube heater, in which steam flows at temperature TS. The rate of heat transfer is given by [itex]\dot{Q} = hA(T_S - T)[/itex]. Fluid entering the tank has a constant temperature of T0, which is also the temperature of the system at t = 0. The differential energy balance for the system is
[tex]\frac{\textrm{d} U}{\textrm{d} t} = \dot{Q} - w \Delta H[/tex]
In terms of temperature
[tex]m C_p \frac{\textrm{d} T}{\textrm{d} t} = hA(T_S - T) - w C_p (T-T_0)[/tex]
The problem only tells you the non-dimensional parameters you are supposed to use
[tex]\Theta = \frac{T-T_0}{T_S - T_0}[/tex]
[tex]\tau = \frac{w}{m} t[/tex]
[tex]\beta = \frac{hA}{w C_p}[/tex]
Which are temperature, time, and an additional constant parameter. In order to start scaling the equation I decided to divide the entire equation by wCp(TS-T0). Also, I've seen some heat transfer textbooks use the relation dT = d(T-T0), because T0 is a constant, so I decided to apply said relation and ended up with the following equation
[tex]\frac{m C_p}{w C_p} \frac{\textrm{d}}{\textrm{d} t} \left( \frac{T-T_0}{T_S-T_0} \right) = \frac{hA(T_S - T)}{w C_p (T_S - T_0)} - \frac{w C_p (T-T_0)}{w C_p (T_S - T_0)}[/tex]
[tex]\frac{m}{w} \frac{1}{\frac{m}{w}} \frac{\textrm{d} \Theta}{\textrm{d} \tau} = \beta \frac{T_S - T}{T_S - T_0} - \Theta[/tex]
Using
[tex]\frac{T_S - T}{T_S - T_0} = 1 - \Theta[/tex]
We get the non-dimensionalized energy balance
[tex]\frac{\textrm{d} \Theta}{\textrm{d} \tau} = \beta (1- \Theta) - \Theta[/tex]
[tex]\frac{\textrm{d} \Theta}{\textrm{d} \tau} = \beta - (\beta +1) \Theta[/tex]
In my opinion, it is not necessary to non-dimensionalize the original D.E. in order to solve it, however, I guess it's a good training problem for non-dimensionalization. I was hoping it could get some insights about how to perform the non-dimensionalization procedure more efficiently. Also, I was wondering, if one is not given the non-dimensional parameters to use, is there a set of rules for choosing them or is it just common sense and practice?
Thanks in advance for any input!