- #1
gracy
- 2,486
- 83
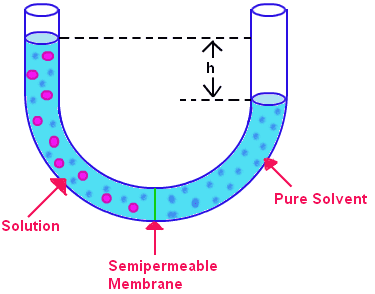
osmotic pressure is the pressure required to stop the flow of solvent molecules due to osmosis.So in case of U shaped tube as in the image below hydrostatic pressure is the pressure which will stop the flow of solvent molecules due to osmosis. So hydrostatic pressure =osmotic pressure right?And if yes is it only in case of u-shaped tube?
http://biology-forums.com/index.php?action=dlattach;topic=196955.0;attach=45336;image
http://biology-forums.com/index.php?action=dlattach;topic=196955.0;attach=45336;image