praveenncyr
- 7
- 0
Hello everyone,
I am very interested in knowing the relation to determine the pressure required to push the water upstream.
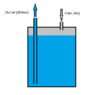
In the attached picture, water is filled inside a tank of volume V and air is constantly flowing inside the tank through an inlet with constant flow rate M. Water should be discharged with a flow rate of W through the outlet. The outlet is maintained at a pressure of P2. Now, considering the fact that air is compressible and temperature dependent, how could I calculate the pressure required by air at the top of water to push the water out through the outlet?
I am sorry for not being so specific about the dimensions. I just want to relate it somehow! Your comments are highly appreciated!
I am very interested in knowing the relation to determine the pressure required to push the water upstream.
In the attached picture, water is filled inside a tank of volume V and air is constantly flowing inside the tank through an inlet with constant flow rate M. Water should be discharged with a flow rate of W through the outlet. The outlet is maintained at a pressure of P2. Now, considering the fact that air is compressible and temperature dependent, how could I calculate the pressure required by air at the top of water to push the water out through the outlet?
I am sorry for not being so specific about the dimensions. I just want to relate it somehow! Your comments are highly appreciated!
Attachments
Last edited by a moderator:
 !
!
 ... its not a state secret project...I don't have specific values...I just want to know how to solve it!
... its not a state secret project...I don't have specific values...I just want to know how to solve it!