Saptarshi Sarkar
- 98
- 13
- TL;DR
- What is the reason behind the exponential part of the well of a proton in the fermi gas model
I was reading an introductory text on nuclear models and came across the Fermi Gas model. I understand that the depth of the potential well of the proton should be less than the depth of the potential well of the neutron due to the Coulombic repulsion between the protons.
But I did not understand the reason behind the red part of the potential in the following diagram.
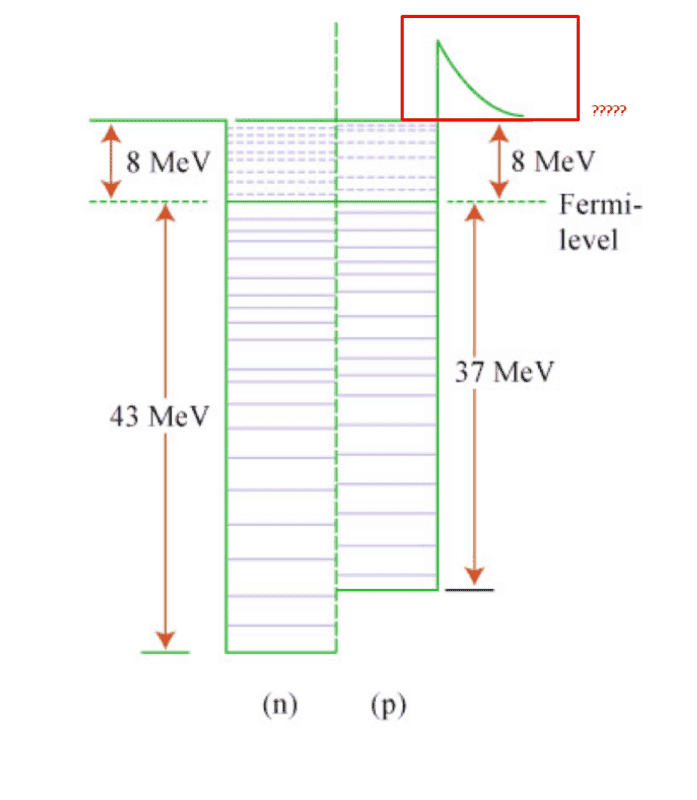
To me, it looks like the red part shows that it is more difficult to pull out a proton from the nucleus that a neutron. But that shouldn't be true as the Coulombic repulsion should make it easier.
But I did not understand the reason behind the red part of the potential in the following diagram.
To me, it looks like the red part shows that it is more difficult to pull out a proton from the nucleus that a neutron. But that shouldn't be true as the Coulombic repulsion should make it easier.