SUMMARY
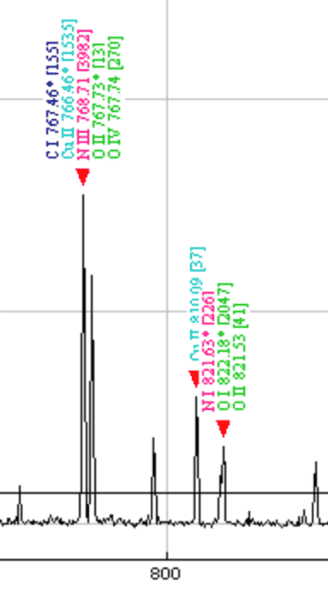
This discussion clarifies the interpretation of plasma spectrum data from a copper plate, specifically addressing the meaning of Roman numerals and bracketed numbers. The Roman numerals (I, II, III) indicate the ionization states of atoms, with I representing a neutral atom, II indicating one electron removed, and III signifying two electrons removed. Additionally, the presence of multiple element names on a single peak is due to closely spaced wavelengths, which may represent individual lines or combinations thereof. The numbers in brackets likely denote the strength of these spectral lines.
PREREQUISITES
- Understanding of plasma spectroscopy
- Familiarity with atomic ionization states
- Knowledge of spectral line intensity
- Basic grasp of wavelength and intensity axes in spectrometry
NEXT STEPS
- Research atomic spectroscopy techniques
- Learn about the significance of spectral line strength
- Explore the concept of ionization energy in elements
- Investigate the use of spectrometers for plasma analysis
USEFUL FOR
Researchers, physicists, and chemists working with plasma spectroscopy, as well as students seeking to understand spectral data interpretation.