Discussion Overview
The discussion revolves around determining the stereochemistry of a reaction product involving stereocenters and the mechanism of an E1 elimination reaction. Participants seek clarification on how to represent stereochemistry and the implications of reaction conditions on the mechanism.
Discussion Character
- Technical explanation
- Conceptual clarification
- Homework-related
- Debate/contested
Main Points Raised
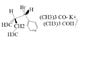
- One participant seeks assistance with writing the reaction product and understanding the stereochemistry represented by wedge and dash notation.
- Another participant requests additional details or images to better understand the initial query.
- A participant identifies that there are two stereocenters and suggests arranging them with hydrogens as dashed, determining priority order for stereochemistry notation.
- A different participant proposes that the reaction is likely an E1 elimination due to the nature of the base and solvent, explaining the reasoning behind this conclusion.
- Participants express gratitude for the assistance provided throughout the discussion.
Areas of Agreement / Disagreement
There is no consensus on the specific stereochemical outcome of the reaction, as participants are still exploring the details of the stereocenters and the elimination mechanism. Multiple views on the reaction mechanism exist, particularly regarding E1 versus E2 elimination.
Contextual Notes
Participants have not fully resolved the assumptions regarding the stereochemistry and the specific reaction conditions that influence the mechanism. The discussion includes varying interpretations of the reaction pathway based on the substrate and solvent used.
Who May Find This Useful
This discussion may be useful for students or individuals interested in organic chemistry, particularly those studying stereochemistry and reaction mechanisms.