Ethan Cheng
- 1
- 0
Hi, I'm currently taking Chemistry 101 and came across this equation that seems to contradict what I've learned before. I don't know the name of it, but here is the equation and its implication.

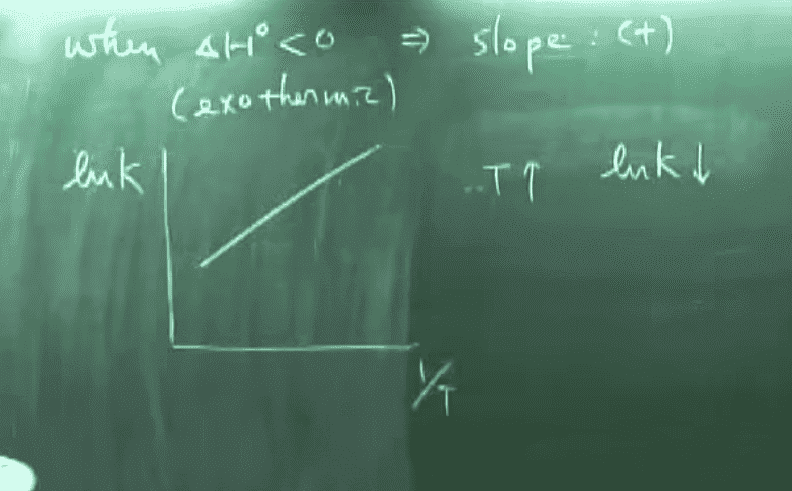
Now another equation we have learned is the Arrhenius equation, which is as follows:
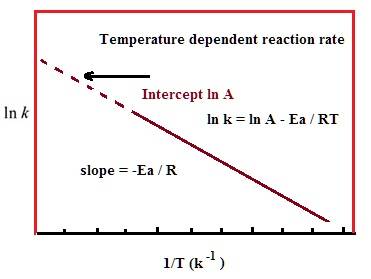
If I understood the equations correctly, they are referring to the same k (equilibrium constant) and the same t (temperature), which gave rise to something that seems to be wrong.
Take the example of combustion, where the activation energy is above 0 and is an exothermic reaction. The graph produced by the first equation will be one with a positive slope, but the one produced by the second equation is one with a negative slope, even though they have the same axis. To illustrate this further, if we somehow experimentally determined the ln k and 1/t (appears very often in our homework), we usually start by graphing them. The linear regression is seen (using Arrhenius equation) as -Ea/R, and we can, therefore, use it to find activation energy. But this same slope should also be -H / R, and therefore will lead to Ea = H, which is also obviously wrong.
What am I missing here? Are the K referring to different values?
Now another equation we have learned is the Arrhenius equation, which is as follows:
If I understood the equations correctly, they are referring to the same k (equilibrium constant) and the same t (temperature), which gave rise to something that seems to be wrong.
Take the example of combustion, where the activation energy is above 0 and is an exothermic reaction. The graph produced by the first equation will be one with a positive slope, but the one produced by the second equation is one with a negative slope, even though they have the same axis. To illustrate this further, if we somehow experimentally determined the ln k and 1/t (appears very often in our homework), we usually start by graphing them. The linear regression is seen (using Arrhenius equation) as -Ea/R, and we can, therefore, use it to find activation energy. But this same slope should also be -H / R, and therefore will lead to Ea = H, which is also obviously wrong.
What am I missing here? Are the K referring to different values?


