- #1
BOAS
- 552
- 19
Hello,
i'm doing my prep for my lab experiment tomorrow and have a few questions about the experiment.
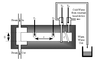
The schematic is attached.
My lab script says the following;
2 questions.
It says the precise rate of flow of water through the heat exchanger is unnecessary to know. Is this because I will be collecting all the water so will know it's mass?
What is the purpose of the constant level cistern? I think this might become clearer when I actually see the apparatus but i'd sleep easier tonight knowing that I understand it's purpose.
Thanks for any help you can give!
i'm doing my prep for my lab experiment tomorrow and have a few questions about the experiment.
The schematic is attached.
My lab script says the following;
Once the steam is flowing well and thermometer T1 has started to rise significantly, adjust the rate of water flow through the heat exchanger until it flows out of the outlet at a few hundred cm3 in 3-5 mins (the precise rate does not matter). The flow rate should be such that the constant level cistern stays filled up to the level of the overflow pipe, but without a huge amount overflowing.
2 questions.
It says the precise rate of flow of water through the heat exchanger is unnecessary to know. Is this because I will be collecting all the water so will know it's mass?
What is the purpose of the constant level cistern? I think this might become clearer when I actually see the apparatus but i'd sleep easier tonight knowing that I understand it's purpose.
Thanks for any help you can give!