SUMMARY
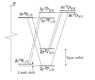
The discussion addresses the absence of an allowed electric dipole transition from the 2s² S1/2 state to the 2p² P3/2 state in hydrogen. The selection rules, specifically Δl = ±1 and Δj = 0, ±1, appear to be satisfied. However, the transition is not observed in optical spectra, indicating it likely occurs in the radio frequency region, which is not represented in the provided diagram.
PREREQUISITES
- Understanding of quantum mechanics and atomic structure
- Familiarity with electric dipole transitions
- Knowledge of selection rules in quantum transitions
- Basic concepts of spectral regions (optical vs. radio frequency)
NEXT STEPS
- Research the implications of selection rules on atomic transitions
- Explore the characteristics of electric dipole transitions in hydrogen
- Investigate the differences between optical and radio frequency transitions
- Study the quantum mechanical treatment of multi-electron atoms
USEFUL FOR
Physicists, particularly those specializing in atomic and quantum physics, as well as students seeking to deepen their understanding of atomic transitions and selection rules.