bznm
- 181
- 0
I've just begun to study spectroscopy, and I need a clarification about the Doppler Effect.
Consider a cell containing Rubidium and enlight it with a laser. Connect the system with an oscilloscope and give a triangular wave as input (so you can know when the Rubidium is resonant). This is the signal that you see downward in the graph. You can see the output in the upper signal.
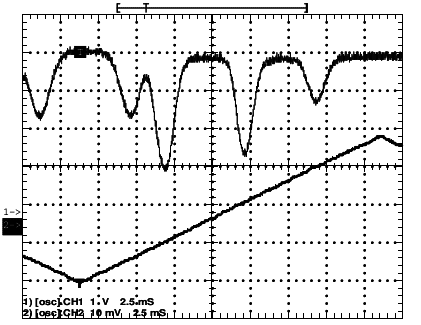
could you explain me how I can see the doppler shift related to the deeper peak and how I can calculate it?
I'm confused because I haven't a course of Spectroscopy and this aspect was treated very very quickly in another course... is the doppler shift the inverse of the FWHM of the peak?
Consider a cell containing Rubidium and enlight it with a laser. Connect the system with an oscilloscope and give a triangular wave as input (so you can know when the Rubidium is resonant). This is the signal that you see downward in the graph. You can see the output in the upper signal.
could you explain me how I can see the doppler shift related to the deeper peak and how I can calculate it?
I'm confused because I haven't a course of Spectroscopy and this aspect was treated very very quickly in another course... is the doppler shift the inverse of the FWHM of the peak?
Last edited: