chiyakotiten
- 3
- 0
Only a substitution product is obtained when the compound below is treated with sodium methoxide. Draw the substitution product and explain why an elimination product is not obtained
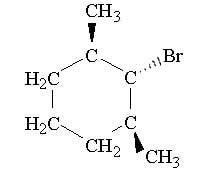
(If the image doesn't show up, the compound is 1-bromo-2,6-dimethylcyclohexane)
- The compound is a secondary structure
- It is going to be an SN2 reaction(?)
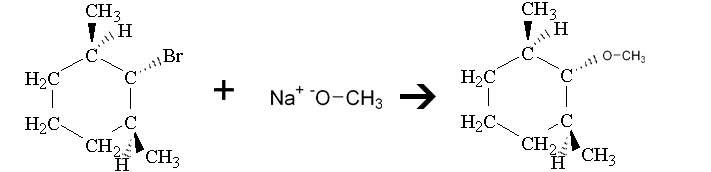
Is it because sodium methoxide is a weak base, but a good nucleophile which favors SN2 reactions?
(If the image doesn't show up, the compound is 1-bromo-2,6-dimethylcyclohexane)
Homework Equations
- The compound is a secondary structure
- It is going to be an SN2 reaction(?)
The Attempt at a Solution
Is it because sodium methoxide is a weak base, but a good nucleophile which favors SN2 reactions?