gregorspv
- 6
- 0
- Homework Statement
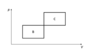
- An ideal monoatomic gas (ϰ=5/3) is repeating a thermodynamic cycle B, which is made out of two isobaric and two isochoric parts. The same gas is repeating a similar cycle C. Both cycles have the same ΔP and ΔV. Express cycle B's efficiency in terms of the efficiency of cycle A.
- Relevant Equations
- Q=mcΔT
η=A/Qin
dA=PdV
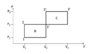
The first picture was provided along the problem statement. The second has my annotations.
I initially began by calculating the ratio of efficiencies, since the work done is obviously the same and cancels out, but after failing and having seen the form of the solution I saw that that cannot work. Therefore I first derived the expression for the efficiency of cycle B: $$\eta_B=\frac{A}{Q_{in,B}}=\frac{\Delta P\Delta V}{mc_V(T_2-T_1)+mc_P(T_{1'}-T_2)}=\frac{\Delta P\Delta V}{mc_V(T_1\frac{P_2}{P_1}-T_1)+mc_P(T_2\frac{V_2}{V_1}-T_2)}=\frac{\Delta P\Delta V}{mc_VT_1(\frac{P_2}{P_1}-1)+mc_PT_1\frac{P_2}{P_1}(\frac{V_2}{V_1}-1)}=\frac{\Delta P\Delta V}{mT_1}\frac{1}{c_V(\frac{P_2}{P_1}-1)+c_P\frac{P_2}{P_1}(\frac{V_2}{V_1}-1)}$$
and similarly
$$\eta_C=\frac{A}{Q_{in,C}}=\frac{\Delta P\Delta V}{mc_V(T_{2'}-T_{1'})+mc_P(T_{3}-T_{2'})}=\frac{\Delta P\Delta V}{mc_VT_1\frac{P_2}{P_1}\frac{V_2}{V_1}(\frac{P_3}{P_2}-1)+mc_P\frac{P_3}{P_1}\frac{V_2}{V_1}(\frac{V_3}{V_2}-1)}=\frac{1}{mT_1}\frac{1}{c_V\frac{V_2}{V_1}\frac{1}{P_1\Delta V}+c_P\frac{P_2}{P_1}\frac{1}{V_1\Delta P}+c_P\frac{1}{P_1V_1}}$$
I then substituted ##\eta_B## into ##\eta_C## to obtain: $$\eta_C=\eta_B(\frac{1}{P_1\Delta V}+\frac{\kappa}{V_1 \Delta P}+\frac{\kappa}{P_1V_1})\frac{1}{\frac{V_2}{V_1}\frac{1}{P_1\Delta V}+\frac{P_2}{P_1}\frac{\kappa}{V_1 \Delta P}+\frac{\kappa}{P_1V_1}}=\eta_B\frac{1}{1+\frac{\Delta V\Delta P+\kappa\Delta P\Delta V}{V_1\Delta P + \kappa P_1\Delta V + \kappa \Delta V\Delta P}}$$
The solution I was given is: $$\eta_C=\eta_B\frac{1}{1+4\eta_B}$$ but this seems incompatible with my derivation. I should suppose there is a much simpler way of approaching this problem?
I initially began by calculating the ratio of efficiencies, since the work done is obviously the same and cancels out, but after failing and having seen the form of the solution I saw that that cannot work. Therefore I first derived the expression for the efficiency of cycle B: $$\eta_B=\frac{A}{Q_{in,B}}=\frac{\Delta P\Delta V}{mc_V(T_2-T_1)+mc_P(T_{1'}-T_2)}=\frac{\Delta P\Delta V}{mc_V(T_1\frac{P_2}{P_1}-T_1)+mc_P(T_2\frac{V_2}{V_1}-T_2)}=\frac{\Delta P\Delta V}{mc_VT_1(\frac{P_2}{P_1}-1)+mc_PT_1\frac{P_2}{P_1}(\frac{V_2}{V_1}-1)}=\frac{\Delta P\Delta V}{mT_1}\frac{1}{c_V(\frac{P_2}{P_1}-1)+c_P\frac{P_2}{P_1}(\frac{V_2}{V_1}-1)}$$
and similarly
$$\eta_C=\frac{A}{Q_{in,C}}=\frac{\Delta P\Delta V}{mc_V(T_{2'}-T_{1'})+mc_P(T_{3}-T_{2'})}=\frac{\Delta P\Delta V}{mc_VT_1\frac{P_2}{P_1}\frac{V_2}{V_1}(\frac{P_3}{P_2}-1)+mc_P\frac{P_3}{P_1}\frac{V_2}{V_1}(\frac{V_3}{V_2}-1)}=\frac{1}{mT_1}\frac{1}{c_V\frac{V_2}{V_1}\frac{1}{P_1\Delta V}+c_P\frac{P_2}{P_1}\frac{1}{V_1\Delta P}+c_P\frac{1}{P_1V_1}}$$
I then substituted ##\eta_B## into ##\eta_C## to obtain: $$\eta_C=\eta_B(\frac{1}{P_1\Delta V}+\frac{\kappa}{V_1 \Delta P}+\frac{\kappa}{P_1V_1})\frac{1}{\frac{V_2}{V_1}\frac{1}{P_1\Delta V}+\frac{P_2}{P_1}\frac{\kappa}{V_1 \Delta P}+\frac{\kappa}{P_1V_1}}=\eta_B\frac{1}{1+\frac{\Delta V\Delta P+\kappa\Delta P\Delta V}{V_1\Delta P + \kappa P_1\Delta V + \kappa \Delta V\Delta P}}$$
The solution I was given is: $$\eta_C=\eta_B\frac{1}{1+4\eta_B}$$ but this seems incompatible with my derivation. I should suppose there is a much simpler way of approaching this problem?