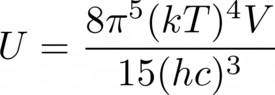Homework Help Overview
The discussion revolves around the pressure-entropy relationship for a photon gas as presented in a problem from Thermal Physics by Daniel Schroeder. The original poster seeks to understand the implications of taking partial derivatives of the internal energy with respect to volume while considering the appropriate variables to hold constant.
Discussion Character
- Conceptual clarification, Mathematical reasoning, Assumption checking
Approaches and Questions Raised
- Participants explore the relationship between internal energy, pressure, and entropy, questioning the conditions under which variables can be held constant. There is a focus on the implications of varying temperature and the nature of photon number in the context of the problem.
Discussion Status
Some participants have provided insights into the relationships between the variables involved, noting the need to keep certain variables constant while deriving expressions. There is an ongoing exploration of the mathematical relationships and assumptions related to the properties of a photon gas.
Contextual Notes
Participants discuss the implications of the first law of thermodynamics and the nature of photon gases, particularly regarding the non-conservation of photon number and its effects on the derivation of pressure from internal energy.